WikiDer > Melanoma
| Melanoma | |
|---|---|
| Boshqa ismlar | Xatarli melanoma |
 | |
| Taxminan 2,5 sm (1 dyuym) dan 1,5 sm (0,6 dyuym) gacha bo'lgan melanoma | |
| Talaffuz | |
| Mutaxassisligi | Onkologiya va dermatologiya |
| Alomatlar | Mole hajmi kattalashib, tartibsiz qirralari, rangi, qichishi yoki o'zgarishi terining buzilishi.[1] |
| Sabablari | Ultraviyole nur (Quyosh, sarg'ish moslamalari)[2] |
| Xavf omillari | Oila tarixi, ko'plab mollar, immunitetning yomonligi[1] |
| Diagnostika usuli | To'qimalarning biopsiyasi[1] |
| Differentsial diagnostika | Seboreik keratoz, lentigo, ko'k nevus, dermatofibroma[3] |
| Oldini olish | Quyosh kremi, ultrabinafsha nurlaridan saqlanish[2] |
| Davolash | Jarrohlik[1] |
| Prognoz | Besh yillik hayot darajasi AQShda 99% (mahalliylashtirilgan), 25% (tarqatilgan)[4] |
| Chastotani | 3,1 million (2015)[5] |
| O'limlar | 59,800 (2015)[6] |
Melanoma, shuningdek, nomi bilan tanilgan malign melanoma, bir turi teri saratoni dan rivojlanadigan pigmentdeb nomlanuvchi hujayralarni ishlab chiqarish melanotsitlar.[1] Melanomalar odatda terida paydo bo'ladi, ammo kamdan-kam hollarda og'izda, ichakda yoki ko'zda paydo bo'lishi mumkin (uveal melanoma).[1][2] Ayollarda ular ko'pincha oyoqlarda, erkaklarda esa ko'pincha orqa tomonda uchraydi.[2] Melanomalarning taxminan 25% dan rivojlanadi mollar.[2] Molning melanomani ko'rsatishi mumkin bo'lgan o'zgarishlar hajmi kattalashishi, qirralarning notekisligi, rangi o'zgarishi, qichishish yoki terining buzilishi.[1]
Melanomaning asosiy sababi bu ultrabinafsha nur (UB) ning darajasi past bo'lganlarda teri pigmenti melanin.[2][7] UV nurlari quyoshdan yoki boshqa manbalardan, masalan sarg'ish moslamalari.[2] Ko'p mollari bo'lganlar, ta'sirlangan oila a'zolari tarixi va immunitetning yomonligi katta xavf ostida.[1] Bir qator noyob genetik sharoit kabi xeroderma pigmentozum shuningdek, xavfni oshiradi.[8] Tashxis qo'yilgan biopsiya va potentsial saraton kasalligi belgilariga ega bo'lgan terining har qanday zararlanishini tahlil qilish.[1]
Foydalanish quyosh kremi va ultrabinafsha nurlaridan saqlanish melanomani oldini oladi.[2] Davolash odatda jarrohlik yo'li bilan olib tashlanadi.[1] Saraton kasalligi biroz kattaroq bo'lganlarda, yaqin atrofda limfa tugunlari tarqalishi uchun sinovdan o'tkazilishi mumkin (metastaz).[1] Agar tarqalish sodir bo'lmasa, ko'p odamlar davolanadi.[1] Melanoma tarqaladiganlar uchun, immunoterapiya, biologik terapiya, radiatsiya terapiyasi yoki kimyoviy terapiya yashashni yaxshilashi mumkin.[1][9] Davolash bilan besh yillik hayot darajasi Qo'shma Shtatlarda mahalliy kasallik bilan kasallanganlar orasida 99%, kasallik limfa tugunlariga tarqalganda 65% va uzoqdan tarqaladiganlar orasida 25%.[4] Melanomaning takrorlanish yoki tarqalish ehtimoli unga bog'liq qalinligi, hujayralar qanchalik tez bo'linadi va ortiqcha terining parchalanishi yoki yo'qligi.[2]
Melanoma terining eng xavfli saraton turi hisoblanadi.[2] Jahon miqyosida, 2012 yilda bu yangi 232000 kishida sodir bo'ldi.[2] 2015 yilda faol kasalliklarga chalingan 3,1 million odam bor edi, natijada 59,800 kishi o'limga olib keldi.[5][6] Avstraliya va Yangi Zelandiyada melanoma darajasi dunyoda eng yuqori ko'rsatkichga ega.[2] Shimoliy Evropa va Shimoliy Amerikada ham yuqori ko'rsatkichlar mavjud, bu Osiyo, Afrika va Lotin Amerikasida kamroq uchraydi.[2] Qo'shma Shtatlarda melanoma erkaklarda ayollarga qaraganda 1,6 marta tez-tez uchraydi.[10] Melanoma 1960 yildan beri asosan aholi yashaydigan hududlarda keng tarqalgan Evropadan chiqqan odamlar.[2][8]
Belgilari va alomatlari
Melanomaning dastlabki belgilari mavjud shakli yoki rangidagi o'zgarishlardir mollar yoki taqdirda tugunli melanoma, terining har qanday joyida yangi shish paydo bo'lishi. Keyingi bosqichlarda mol bo'lishi mumkin qichima, oshqozon yarasi yoki qon ketish. Melanomaning dastlabki belgilari "ABCDEF" mnemonikasi bilan umumlashtiriladi:[11][12]
- Asimmetriya
- Bbuyurtmalar (chekkalari va burchaklari bilan tartibsiz)
- Crang (rang-barang)
- D.iametr (6 mm dan katta (0,24yilda), qalam silgi hajmiga teng)
- Evaqt o'tishi bilan volving
- Fyoqimsiz qarab
Ushbu tasnif o'z tasniflariga ega bo'lgan tugunli melanomaga taalluqli emas:[13]
- Eteri yuzasidan yuqorida joylashgan
- Fteginish uchun irm
- Geshkak eshish
Metastatik melanoma nonspesifik sabab bo'lishi mumkin paraneoplastik alomatlarshu jumladan ishtahani yo'qotish, ko'ngil aynish, qusish va charchoq. Metastaz Erta melanomaning tarqalishi (tarqalishi) mumkin, ammo nisbatan kam uchraydi: erta tashxis qo'yilgan melanomalarning beshdan bir qismidan kamrog'i metastatik bo'ladi. Miya metastazlari metastatik melanomali bemorlarda ayniqsa keng tarqalgan.[14] Shuningdek, u jigar, suyaklar, qorin yoki uzoq limfa tugunlariga tarqalishi mumkin.
Sababi
Melanomalar odatda quyoshning ultrabinafsha nurlari ta'sirida DNKning shikastlanishidan kelib chiqadi. Genetika ham rol o'ynaydi.[15][16] Melanoma, shuningdek, quyosh nurlari kam bo'lgan teri joylarida (ya'ni og'iz, oyoq taglari, kaftlar, jinsiy a'zolar) paydo bo'lishi mumkin.[17] Odamlar displastik nevus sindromi, shuningdek, oilaviy atipik ko'p sonli melanoma (FAMMM) deb nomlanuvchi melanoma rivojlanish xavfi yuqori.[18]
Ellikdan ortiq molga ega bo'lish melanoma xavfi oshishi mumkinligini ko'rsatadi. Immunitet tizimining zaiflashishi organizmning saraton hujayralari bilan kurashish qobiliyati sustligi tufayli saraton paydo bo'lishini osonlashtiradi.[15]
UV nurlanishi
Ko'nchilik yotoqlarining ultrabinafsha nurlanishi melanoma xavfini oshiradi.[19] The Xalqaro saraton tadqiqotlari agentligi ko'nchilik yotoqlari "odamlar uchun kanserogen" ekanligini va o'ttiz yoshga to'lmasdan terining terisini ishlatishni boshlagan odamlarning melanomaga chalinish ehtimoli 75% yuqori ekanligini aniqlaydi.[20]
Samolyotlarda ishlaydiganlar, shuningdek, ultrabinafsha nurlari ta'siriga bog'liq deb taxmin qilishadi.[21]
Ultraviyole UVB nurlari (to'lqin uzunligi 315 dan 280 nm gacha) quyoshdan teri hujayralari DNKsi tomonidan so'riladi va natijada to'g'ridan-to'g'ri DNKning shikastlanishi deb nomlangan siklobutan pirimidin dimerlari (CPD). Timin-timin, sitozin-sitozin yoki sitozin-timin dimerlar ikkita qo'shni qo'shilish orqali hosil bo'ladi pirimidin DNK zanjiri ichidagi asoslar. Bir oz o'xshash UVB, UVA nurlari (400 dan 315 nm gacha bo'lgan to'lqin uzunliklari) quyoshdan yoki qalbakilashtirilgan teridan to'g'ridan-to'g'ri terining DNKsi tomonidan so'rilishi mumkin (UVB so'rilganidan 100-1000 baravar past samaradorlikda).[22]
Himoyasizlik ultrabinafsha nurlanish (UVA va UVB) melanoma rivojlanishiga katta hissa qo'shadi.[23] Vaqti-vaqti bilan haddan tashqari quyosh nurlari (natijada "quyosh yonishi") melanoma bilan bog'liq.[24] Melanoma ko'pincha erkaklarda orqa tomonda va ayollarda oyoqlarda uchraydi (vaqti-vaqti bilan quyosh ta'sir qiladigan joylar). Xavfga tashqi ish joylariga nisbatan emas, balki ijtimoiy-iqtisodiy sharoitlar kuchli ta'sir ko'rsatmoqda; bu malakasiz ishchilarga qaraganda professional va ma'muriy ishchilarda ko'proq uchraydi.[25][26] Boshqa omillar mutatsiyalar ichida yoki umuman yo'qotish o'smani bostiruvchi genlar. Dan foydalanish choyshablar (chuqur penetratsion UVA nurlari bilan) teri saratoni, shu jumladan melanoma rivojlanishiga bog'liq.[27]
Xavfni aniqlashning mumkin bo'lgan muhim elementlari orasida quyoshga ta'sir qilishning intensivligi va davomiyligi, quyosh nurlari paydo bo'lish yoshi va darajasi kiradi teri pigmentatsiyasi. Melanoma darajasi migrantlar tomonidan joylashtirilgan mamlakatlarda eng yuqori ko'rsatkichga ega shimoliy Evropa ko'p miqdordagi to'g'ridan-to'g'ri, kuchli quyosh nuriga ega, bu ko'chmanchilar terisi moslashtirilmagan, xususan Avstraliyaga. Bolalik davrida ta'sirlanish katta yoshdagi ta'sirga qaraganda muhimroq xavf omilidir. Bu Avstraliyadagi migratsiya tadqiqotlarida kuzatiladi.[28]
Ko'p marta kuchli kuyish holatlari, kelajakda kuyishlarning kümülatif zararlanish tufayli melanomaga aylanish ehtimolini oshiradi.[15] Quyosh va bronzalar ultrafiolet nurlanishining asosiy manbalari bo'lib, melanoma xavfini oshiradi va ekvatorga yaqin joyda yashash ultrabinafsha nurlanish ta'sirini oshiradi.[15]
Genetika
Ko'pincha oilalarda uchraydigan bir qator noyob mutatsiyalar melanoma sezuvchanligini sezilarli darajada oshiradi. Bir nechta genlar xatarlarni oshirish. Ba'zi noyob genlarning melanomani keltirib chiqarish xavfi nisbatan yuqori; ba'zi bir keng tarqalgan genlar, masalan, deb nomlangan gen MC1R qizil sochlarni keltirib chiqaradigan xavf darajasi nisbatan past. Genetik sinov mutatsiyalarni qidirishda foydalanish mumkin.
Mutatsiyalarning bir klassi genga ta'sir qiladi CDKN2A. Shu bilan bir qatorda o'qish doirasi bu gendagi mutatsiya beqarorlikni keltirib chiqaradi p53, a transkripsiya omili da ishtirok etish apoptoz odam saratonining ellik foizida. Xuddi shu gendagi yana bir mutatsiya funktsional bo'lmagan inhibitorga olib keladi CDK4, a velosiped- mustaqil kinaz bu targ'ib qiladi hujayraning bo'linishi. Teri holatini keltirib chiqaradigan mutatsiyalar xeroderma pigmentozum (XP) melanoma sezuvchanligini oshiradi. Genom bo'ylab tarqalgan bu mutatsiyalar hujayraning DNKni tiklash qobiliyatini pasaytiradi. CDKN2A va XP mutatsiyalari ikkalasi ham yuqori darajada ta'sir o'tkazuvchidir (tashuvchining fenotipni ifoda etish ehtimoli katta).
Oilaviy melanoma (FAMMM) genetik jihatdan bir jinsli emas,[16] va oilaviy melanoma uchun lokuslar paydo bo'ladi xromosoma qo'llar 1p, 9p va 12q. Ko'plab genetik hodisalar melanoma bilan bog'liq edi patogenez (kasallik rivojlanishi).[29] Ko'p sonli o'simta supressori 1 (CDKN2A / MTS1) geni p16INK4a - pastmolekulyar og'irlik oqsil inhibitori siklinga bog'liq oqsil kinazalari (CDK) - p21 mintaqasiga joylashtirilgan inson xromosomasi 9.[30] FAMMM odatda oilaviy tarixda melanoma bilan bir qatorda 50 yoki undan ortiq birlashtirilgan molga ega bo'lishi bilan tavsiflanadi.[17] U autosomal tarzda dominant tarzda uzatiladi va asosan CDKN2A mutatsiyasiga bog'liq.[17] CDKN2A mutatsiyasiga uchragan FAMMM bo'lgan odamlarda oshqozon osti bezi saratoni xavfi 38 barobar oshadi.[31]
Boshqa mutatsiyalar kamroq xavf tug'diradi, ammo aholi orasida tez-tez uchraydi. Mutatsiyalarga ega odamlar MC1R genning melanoma rivojlanishi ikki yovvoyi turdagi (odatdagi ta'sirlanmagan tip) nusxalariga qaraganda ikki-to'rt baravar ko'p. MC1R mutatsiyalari juda keng tarqalgan; va barcha qizil sochli odamlar mutatsiyaga uchragan nusxaga ega.[iqtibos kerak] Ning mutatsiyasi MDM2 SNP309 geni yosh ayollar uchun xavfning oshishi bilan bog'liq.[32]
Odil va qizil sochli odamlar, ko'p sonli atipik odamlar nevuslar yoki displastik nevuslar va gigant bilan tug'ilgan odamlar tug'ma melanotsitik nevuslar yuqori xavf ostida.[33]
Melanomaning oilaviy tarixi odamning xavfini sezilarli darajada oshiradi, chunki melanomaga moyil bo'lgan oilalarda bir nechta genlarning mutatsiyalari topilgan.[34] Anamnezida bir melanoma bo'lgan odamlarda ikkinchi darajali o'smaning paydo bo'lish xavfi yuqori.[35]
Ochiq teri - bu terida kam melanin bo'lishining natijasidir, ya'ni ultrabinafsha nurlanishidan himoya kamroq bo'ladi.[15] Oilaviy tarix melanomaga genetik moyillikni ko'rsatishi mumkin.[15]
Patofiziologiya

Melanomaning dastlabki bosqichi qachon boshlanadi melanotsitlar nazoratdan tashqari o'sishni boshlash. Melanotsitlar terining tashqi qatlami orasida joylashgan ( epidermis) va keyingi qatlam (the dermis). Kasallikning ushbu dastlabki bosqichi o'smaning qalinligi 1 mm dan kam bo'lganda radial o'sish bosqichi deb ataladi. Saraton hujayralari terining chuqur tomirlariga hali etib bormaganligi sababli, bu dastlabki bosqichdagi melanoma tananing boshqa qismlariga tarqalishi ehtimoldan yiroq emas. Agar ushbu bosqichda melanoma aniqlansa, u holda odatda jarrohlik yo'li bilan to'liq olib tashlanishi mumkin.
Shish hujayralari boshqa yo'nalishda harakat qilishni boshlaganda - vertikal ravishda epidermisga va ichiga papiller dermis - hujayraning harakati keskin o'zgaradi.[36]
Evolyutsiyaning navbatdagi bosqichi - bu hujayralar invaziv potentsialga ega bo'lishni boshlaydigan invaziv radial o'sish bosqichi. Shu vaqtdan boshlab melanoma tarqalishi mumkin. The Breslovning chuqurligi shikastlanish odatda 1 mm dan kam (0,04)yilda), esa Klark darajasi odatda 2 ga teng.
Vertikal o'sish bosqichi (VGP) bu invaziv melanoma. Shish atrofdagi to'qimalarda o'sishi mumkin bo'ladi va tanada qon orqali tarqalishi mumkin limfa tomirlari. Shishning qalinligi odatda 1 mm dan oshadi (0,04.)yilda), va o'sma dermisning chuqur qismlarini o'z ichiga oladi.
Uy egasi VGP paytida o'smaga qarshi immunologik reaktsiyani keltirib chiqaradi,[37] ning mavjudligi va faoliyati bilan baholanadi o'simta infiltratsiyali limfotsitlar (TIL). Ushbu hujayralar ba'zan birlamchi o'smani butunlay yo'q qiladi; bu rivojlanishning so'nggi bosqichi bo'lgan regressiya deb ataladi. Muayyan holatlarda birlamchi o'sma butunlay yo'q bo'lib ketadi va faqat metastatik o'sma aniqlanadi. Odam melanomalarining taxminan 40% B-Raf tuzilishiga ta'sir qiluvchi faol mutatsiyalarni o'z ichiga oladi oqsil, natijada Raf orqali konstitutsiyaviy signal beriladi MAP kinazasi yo'l.[38]
Ko'pgina saraton kasalliklari uchun odatiy haqorat - bu zarar DNK.[39] UVA nurlari asosan sabab bo'ladi timin dimerlari.[40] UVA ham ishlab chiqaradi reaktiv kislorod turlari va ular DNKning boshqa zararlanishiga olib keladi, birinchi navbatda bir qatorli tanaffuslar oksidlanadi pirimidinlar va oksidlangan purin 8-oksoguanin (mutagenli DNK o'zgarishi) navbati bilan 1/10, 1/10 va 1/3 da UVA ta'sirida bo'lgan timin dimerlarining chastotalari.
Agar CPD fotoproduktalari ta'mirlanmagan bo'lsa, mutatsiyalarga noto'g'ri sabab bo'lishi mumkin translesion sintez DNKning replikatsiyasi yoki tiklanishi paytida. O'tgan CPDlarning noto'g'ri sintezi tufayli eng tez-tez uchraydigan mutatsiyalar sitozindan timingacha (C> T) yoki CC> TT hisoblanadi. o'tish mutatsiyalari. Ular odatda UV barmoq izlari deb nomlanadi mutatsiyalar, chunki ular ultrabinafsha nurlari ta'siridagi eng o'ziga xos mutatsiya bo'lib, ular tez-tez quyosh nurlari ta'sir qiladigan terida uchraydi, ammo ichki organlarda kamdan-kam uchraydi.[41] UV fotoelementlarini DNK bilan tiklashdagi xatolar yoki ushbu fotoproduktlardan o'tib ketgan noto'g'ri sintez ham o'chirishga, qo'shimchalarga va olib kelishi mumkin xromosoma translokatsiyalari.
25 ta melanomaning barcha genomlari tartiblangan.[42] O'rtacha melanoma genomiga taxminan 80,000 mutatsiyaga uchragan bazalar (asosan C> T o'tish) va 100 ga yaqin tuzilish o'zgarishlari topilgan. Bu avlodlar bo'ylab (ota-onadan bolaga) taxminan 70 ta mutatsiyadan ancha yuqori.[43][44] 25 ta melanoma orasida taxminan 6000 ta protein kodlovchi gen mavjud edi missense, bema'nilik, yoki qo'shilish joyidagi mutatsiyalar. Shuningdek, 100 dan ortiq melanomalarning transkriptomlari ketma-ketlikda va tahlil qilingan. Odamning barcha protein kodlash genlarining deyarli 70% melanomada ifodalanadi. Ushbu genlarning aksariyati boshqa normal va saraton to'qimalarida ham namoyon bo'ladi, saratonning boshqa shakllariga nisbatan 200 ga yaqin gen melanomada o'ziga xos ekspresyon naqshini namoyish etadi. Melanomaga xos genlarga misollar tirozinaza, MLANAva PMEL.[45][46]
UV nurlanishiga sabab bo'ladi zarar uchun DNK odatda hujayralar timin dimerizatsiya, uni tuzatilmasa yaratishi mumkin mutatsiyalar kamerada genlar. Ushbu kuchli mutagen omil terining melanomasini eng ko'p mutatsiyalarga ega o'sma turiga aylantiradi.[47] Hujayra qachon ajratadi, bu mutatsiyalar hujayralarning yangi avlodlariga tarqaladi. Agar mutatsiyalar sodir bo'lsa protoonkogenlar yoki o'smani bostiruvchi genlar, darajasi mitoz mutatsiyaga ega hujayralarda nazoratsiz bo'lib, a hosil bo'lishiga olib kelishi mumkin o'sma. Bemorlarning ma'lumotlari shuni ko'rsatadiki, melanoma hujayralarining yadrosidagi faollashtiruvchi transkripsiya omilining me'yor darajalari melanoma hujayralarining metastatik faolligining oshishi bilan bog'liq;[48][49][50] sichqonlardan teri saratoni bo'yicha olib borilgan tadqiqotlar saraton rivojlanishida transkripsiya omil-2 ni faollashtirish rolini tasdiqlashga moyildir.[51][52]
Saraton xujayralari ishtirok etishi mumkin.[53]
Gen mutatsiyalari
Kabi keng ko'lamli tadqiqotlar Saraton genomi atlasi takrorlanuvchi xususiyatga ega somatik o'zgarishlar ehtimol teri qo'zg'atuvchisi qo'zg'atuvchisi va rivojlanishi.[54]
Eng tez-tez uchraydigan mutatsiya 600-kodonda uchraydi BRAF (50% holatlar). BRAF odatda hujayra o'sishida ishtirok etadi va bu mutatsiya oqsilni normal faol va normal fiziologik regulyatsiyadan mustaqil qiladi, shu bilan o'smaning o'sishiga yordam beradi.[55] RAS genlari (NRAS, HRAS va KRAS) shuningdek takroriy mutatsiyaga uchraydi (TCGA holatlarining 30%) va 61 yoki 12-kodonlardagi mutatsiyalar onkogen faollikni keltirib chiqaradi. Yo'qotilgan mutatsiyalar ko'pincha ta'sir qiladi o'smani bostiruvchi genlar kabi NF1, TP53 va CDKN2A. Boshqa onkogen o'zgarishlarga BRAF kabi turli kinazlarni o'z ichiga olgan sintezlar kiradi,[56] RAF1,[57] ALK, RET, ROS1, NTRK1.,[58] NTRK3[59] va MET[60] BRAF, RAS, NF1 mutatsiyalari va kinazli termoyadroviylar bir-biridan ajralib turadi, chunki ular bemorlarning turli xil pastki qismlarida uchraydi. Mutatsiya holatini baholash shuning uchun bemorning tabaqalanishini yaxshilashi va aniq inhibitorlar bilan maqsadli terapiyani xabardor qilishi mumkin.
Ba'zi hollarda (3-7%) BRAF va NRASning mutatsiyaga uchragan versiyalari o'tkaziladi raqamni kuchaytirish.[54]
Tashxis


Ko'rib chiqilayotgan hududga qarash melanomaga shubha qilishning eng keng tarqalgan usuli hisoblanadi.[61] Ranglari yoki shakli notekis bo'lgan mollarga odatda nomzod sifatida qaraladi. Melanomalarni aniqlash (va hayot darajasini oshirish) uchun ularni tanib olishni o'rganish tavsiya etiladi (qarang) "ABCDE" mnemonik), muntazam ravishda tekshirish mollar o'zgarishlar uchun (shakli, hajmi, rangi, qichishi yoki qon ketishi) va nomzod paydo bo'lganda malakali shifokor bilan maslahatlashish.[62][63]
Biroq, ko'plab melanomalar diametri 6 mm dan kichik bo'lgan lezyonlar shaklida namoyon bo'ladi; va barcha melanomalar dastlab kichik nuqta bo'lib ko'ringanida xavfli hisoblanadi. Shifokorlar odatda barcha mollarni, shu jumladan diametri 6 mm dan kam bo'lganlarni tekshiradilar. Seboreik keratoz ABCD mezonlarining bir qismiga yoki barchasiga javob berishi mumkin va olib kelishi mumkin yolg'on signalizatsiya. Shifokorlar, odatda, seborik keratozni melanomadan tekshiruv paytida yoki bilan farqlashlari mumkin dermatoskopiya.[iqtibos kerak]
Ba'zilar kattalashtirishni evolyutsiya bilan almashtirishni yoqlaydilar. O'zgaradigan va rivojlanayotgan mollar tashvishga solishi aniq. Shu bilan bir qatorda, ba'zi amaliyotchilar balandlikni afzal ko'rishadi. Balandlik melanomani aniqlashga yordam beradi, ammo balandlikning yo'qligi bu lezyon melanoma emas degani emas. AQShdagi melanomalarning aksariyati ular ko'tarilishidan oldin aniqlanadi. Ko'tarilish ko'rinadigan vaqtga kelib, ular xavfli invaziv bosqichga o'tishlari mumkin.[iqtibos kerak]
Shubhali teri lezyonlarini shaxsan tekshirish shubhali teri lezyonlari tasvirlarini vizual tekshirishdan ko'ra aniqroq.[64] Dermoskopiya o'qitilgan mutaxassislar tomonidan qo'llanilganda, faqat yalang'och ko'zdan foydalanishdan ko'ra, malign lezyonlarni aniqlash foydalidir.[65] Yansıtıcı konfokal mikroskopi terining melanomasini tashxislashda dermoskopiyaga qaraganda yaxshiroq sezgirlik va o'ziga xoslikka ega bo'lishi mumkin, ammo bu natijani tasdiqlash uchun ko'proq tadqiqotlar talab etiladi.[66]
Bilan terining biopsiyasida melanoma H&E binoni - bu holat yuzaki tarqaladigan melanomani ko'rsatishi mumkin.
Yomon o'rdak
Usullardan biri "yomon o'rdak imzo ".[67] Umumiy lezyon xususiyatlarining o'zaro bog'liqligi amalga oshiriladi. Umumiy xususiyatlardan chetga chiqadigan jarohatlar "Xunuk o'rdak" deb belgilanadi va qo'shimcha kasbiy imtihon talab qilinadi. "Qizil qalpoqcha"belgisi[67] ochiq teri va sochlari ochiq bo'lgan odamlarga tashxis qo'yish qiyin bo'lishi mumkinligini ko'rsatadi amelanotik melanomalar. Bunday odamlarni tekshirishda qo'shimcha ehtiyotkorlik talab etiladi, chunki ular bir nechta melanomaga ega bo'lishi mumkin va juda og'ir displastik nevuslar. "Yomon o'rdak" ni aniqlash uchun dermatoskopdan foydalanish kerak, chunki bu odamlarning ko'plab melanomalari melanomalarga o'xshamaydi yoki ular "qo'y kiyimi kiygan bo'rilar".[68] Ushbu adolatli terida ko'pincha engil pigmentli yoki amelanotik melanomalar mavjud bo'lib, ular ranglarning o'zgarishi va o'zgarishini kuzatishi oson emas. Ularning chegaralari ko'pincha noaniq bo'lib, dermatoskopsiz vizual identifikatsiyani murakkablashtiradi.
Odil teri odamlarda paydo bo'lgan amelanotik melanomalar va melanomalarni aniqlash juda qiyin, chunki ular ABCD qoidasidagi ko'plab xususiyatlarni ko'rsatolmaydilar, "Ugly Duckling" belgisini sindirishadi va husnbuzar izlari, hasharotlar chaqishi, dermatofibromalar, yoki lentiginlar.
Biopsiya
Vizual tekshiruv va dermatoskopik tekshiruvdan so'ng,[68] yoki jonli ravishda konfokal mikroskop kabi diagnostika vositalari, shifokor mumkin biopsiya shubhali mol. A teri biopsiyasi ostida ijro etilgan lokal behushlik tez-tez tashxis qo'yish yoki tasdiqlashda va zo'ravonlikni aniqlashda yordam berish uchun talab qilinadi. Elliptik eksizion biopsiya o'smani olib tashlashi mumkin, so'ngra gistologik tahlil va Breslou skorlari. Kabi kesma biopsiya musht biopsiya namuna olishda xato bo'lishi mumkinligi sababli, odatda melanomada gumon qilingan holatlarda kontrendikedir[69] yoki o'simta qalinligini noto'g'ri baholashga olib keladigan mahalliy implantatsiya.[70][71] Ammo, bunday biopsiyalar metastatik kasallik xavfini oshirishi mumkin degan qo'rquv asossiz bo'lib tuyuladi.[72][73]
Imkon qadar tana yuzasining fotosurat hujjatlarini o'z ichiga olgan umumiy tana fotosuratlari, ko'pincha yuqori xavfli bemorlarni kuzatishda qo'llaniladi. Ushbu texnikani erta aniqlashga imkon beradigan va har qanday raqamli kameraga ega bo'lgan holda iqtisodiy jihatdan samarali yondashuvni taqdim etganligi haqida xabar berilgan, ammo uning samaradorligi makroskopik o'zgarishlarni aniqlay olmasligi sababli shubha ostiga olingan.[61] Diagnostika usulini dermoskopik ko'rish bilan birgalikda ishlatish kerak (va uning o'rnini bosuvchi emas), ikkala usulning ham kombinatsiyasi juda yuqori darajada aniqlashga imkon beradi.
Gistopatologik turlari
Melanoma - bu turi neyroektodermal neoplazma.[74] Melanomaning to'rtta asosiy turi mavjud:[75]
| Turi | Xususiyatlari | Hodisa[75][1-qayd] | Fotosurat | Mikrograf |
|---|---|---|---|---|
| Yuzaki tarqaladigan melanoma | Dermo-epidermal birikma bo'ylab uyasi shakllangan melanoma hujayralari. | 70% |  |  |
| Nodüler melanoma | Kenglikdan ko'ra chuqurlikda nisbatan ko'proq o'sadi. | 15% - 20% |  |  |
| Lentigo maligna melanomasi | Atipik epidermal melanotsitlarning chiziqli tarqalishi, shuningdek dermisga kirib borish.[76] | 5% - 10% |  |  |
| Akral lentiginli melanoma | Dermoepidermal birikmada atipik melanotsitlarning doimiy tarqalishi.[77] | 7% - 10% | 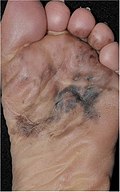 |  |
Boshqa histopatologik turlari:
- Mukozal melanoma; Melanoma paydo bo'lganda shilliq pardalar.
- Desmoplastik melanoma
- Nevusga o'xshash kichik hujayralar bilan melanoma
- Spits nevusining xususiyatlari bilan melanoma
- Uveal melanoma
- Vaginal melanoma
- Polipoid melanoma, tugunli melanoma subklassi.
In situ yoki invaziv
Melanoma joyida tashqarisidan bostirib kirmagan bazal membrana, shu bilan birga invaziv melanoma undan tashqariga tarqaldi.
Melanomaning ba'zi histopatologik turlari o'ziga xos invazivdir, shu jumladan tugunli melanoma va lentigo maligna melanoma, qaerda joyida lentigo maligna melanomasining hamkori lentigo maligna.[78] Lentigo maligna ba'zan juda erta melanoma deb tasniflanadi,[79] va ba'zida melanomaning kashfiyotchisi.[80]
Yuzaki tarqaladigan melanomalar va akral lentiginli melanomalar ham bo'lishi mumkin joyida yoki invaziv,[81] ammo akral lentiginli melanomalar deyarli har doim invazivdir.[82]
Sahnalashtirish
Qo'shimcha kontekst saraton kasalligi mavjud TNM.
Metastatik melanomalarni rentgen nurlari, tomografiya, MRI, PET va PET / KT, ultratovush tekshiruvi, LDH tekshiruvi va fotoakustik aniqlash orqali aniqlash mumkin.[83] Shu bilan birga, melanomaga chalingan odamlarni turli xil tasvirlash usullari bilan sahnalashtirishning aniqligida dalillar yo'q.[84]
Melanoma bosqichlari AJCC, 8-nashr:[85]
- TX: Birlamchi o'smaning qalinligini baholash mumkin emas (masalan, kuretaj orqali tashxis qo'yish)
- T0: Birlamchi o'smaning isboti yo'q (masalan, noma'lum birlamchi yoki to'liq regresslangan melanoma)
| Bosqich | T toifasi[85] | Qalinligi[85] | Oshqozon yarasi[85] |
|---|---|---|---|
| 0 bosqich | Melanoma joyida | ||
| I bosqich | T1a | 0,8 mm dan kam | Yo'q |
| T1b | 0,8 mm dan kam | Yo'q | |
| > 0,8 dan 1,0 mm gacha | Ha | ||
| T2a | > 1,0 dan 2,0 mm gacha | Yo'q | |
| II bosqich | T2b | > 1,0 dan 2,0 mm gacha | Ha |
| T3a | > 2,0 dan 4,0 mm gacha | Yo'q | |
| T3b | > 2,0 dan 4,0 mm gacha | Ha | |
| T4a | > 4,0 mm | Yo'q | |
| T4b | > 4,0 mm | Ha | |
1 va 2 bosqichda N (limfa tugunlari) klassi talab qilinadi:
- N0 - Hududiy metastazlar yo'q.[85]
| Bosqich | N toifasi | Shish bilan bog'liq bo'lgan mintaqaviy limfa tugunlari soni | Tranzit, sun'iy yo'ldosh va / yoki mikrosatellit metastazlarining mavjudligi |
|---|---|---|---|
| Yo'q | NX | Mintaqaviy tugunlar baholanmagan (masalan, sentinel limfa tugunlari biopsiyasi o'tkazilmagan yoki boshqa sabablarga ko'ra ilgari olib tashlangan mintaqaviy tugunlar)[2-qayd] | |
| III bosqich | N1 | Ulardan biri limfa tugunlari yoki har qanday tranzit, sun'iy yo'ldosh va / yoki mikrosatellit metastazlari, o'smalarga aloqador tugunlarsiz. | |
| N1a | Bittasi klinik jihatdan yashirin (ya'ni sentinel tugun biopsiyasi bilan aniqlanadi) | Yo'q | |
| N1b | Ulardan biri klinik jihatdan aniqlangan | Yo'q | |
| N1c | Mintaqaviy limfa tugunlari kasalligi yo'q | Ha | |
| N2 | Ikki yoki uchta shish paydo bo'lgan tugunlar yoki bir nechta tranzit, sun'iy yo'ldosh va / yoki mikrosatellit metastazlari, bitta o'sma bilan bog'langan tugun | ||
| N2a | Ikki yoki uchta klinik sir (ya'ni, qo'riqchi tugunlari biopsiyasi bilan aniqlanadi) | Yo'q | |
| N2b | Ikkita yoki uchta, ulardan kamida bittasi klinik jihatdan aniqlangan | Yo'q | |
| N2c | Klinik ravishda yashirin yoki klinik jihatdan aniqlangan | Ha | |
| N3 | To'rt yoki undan ortiq o'sma bilan bog'langan tugunlar yoki tranzit, sun'iy yo'ldosh va / yoki mikrosatellit metastazlarining har qanday soni 2 yoki undan ortiq o'sma bilan bog'langan tugunlar yoki har qanday tirnoqli tugunlar, tranzit, sun'iy yo'ldosh va / yoki mikrosatellitsiz yoki bo'lmagan holda. metastazlar | ||
| N3a | To'rt yoki undan ortiq klinik okkult (ya'ni, qo'riqchi tugunlari biopsiyasi bilan aniqlanadi) | Yo'q | |
| N3b | To'rtta yoki undan ko'prog'i, ulardan kamida bittasi klinik jihatdan aniqlangan yoki har qanday moslashtirilgan tugunlarning mavjudligi | Yo'q | |
| N3c | Ikki yoki undan ortiq klinik jihatdan yashirin yoki klinik jihatdan aniqlangan va / yoki har qanday moslashtirilgan tugunlarning mavjudligi | Ha | |
1, 2 va 3 bosqichda M (metastaz holati) talab qilinadi:
- M0: Uzoq metastaz haqida ma'lumot yo'q
| Bosqich | M toifasi | Anatomik sayt | laktat dehidrogenaza (LDH) darajasi |
|---|---|---|---|
| IV bosqich | M1 | Uzoq metastazning dalillari | |
| M1a | Teriga, yumshoq to'qimalarga, shu jumladan mushaklarga va / yoki mintaqaviy bo'lmagan limfa tugunlariga uzoq metastaz | Yozilmagan yoki aniqlanmagan | |
| M1a (0) | Baland emas | ||
| M1a (1) | Baland | ||
| M1b | M1a joylarida metastazli yoki metastazsiz o'pkaga uzoq metastaz | Yozilmagan yoki aniqlanmagan | |
| M1b (0) | Baland emas | ||
| M1b (1) | Baland | ||
| M1c | M1a yoki M1b joylariga metastazli yoki bo'lmagan holda CNS bo'lmagan visseral joylarga masofadan metastaz. | Yozilmagan yoki aniqlanmagan | |
| M1c (0) | Baland emas | ||
| M1c (1) | Baland | ||
| M1d | M1a, M1b yoki M1c joylariga metastazli yoki metastazisiz CNSgacha bo'lgan metastaz. | Yozilmagan yoki aniqlanmagan | |
| M1d (0) | Baland emas | ||
| M1d (1) | Baland | ||
Eski tizimlarga "Klark darajasi"va"Breslovning chuqurligi", o'sma invaziyasining mikroskopik chuqurligini miqdoriy aniqlash.
Laboratoriya
Laktat dehidrogenaza (LDH) testlari ko'pincha skrining uchun ishlatiladi metastazlar, metastazli ko'plab bemorlarda (hatto oxirgi bosqichda) normal LDH bo'lsa ham; favqulodda yuqori LDH ko'pincha kasallikning jigarga metastatik tarqalishini ko'rsatadi.
Odatda melanoma tashxisi qo'yilgan bemorlarda ko'krak qafasi rentgenogrammasi va LDH testi, ba'zida esa KT, MRI, UY HAYVONIva / yoki PET / KT tekshiruvlari. Garchi bahsli bo'lsa ham, qo'riqchi limfa tuguni biopsiya va limfa tugunlari limfa tugunlariga tarqalishini baholash uchun bemorlarda ham amalga oshiriladi. Melanoma tashxisi mavjud bo'lganligi bilan tasdiqlanadi S-100 oqsili marker.
HMB-45 melanomalar kabi melanotsitik o'smalarda mavjud bo'lgan antigenga qarshi reaksiyaga kirishadigan monoklonal antikor. Anatomik patologiyada bunday o'smalar uchun marker sifatida ishlatiladi. Antikor melanoma ekstrakti uchun hosil bo'ldi. U melanotsitik o'smalarga ijobiy ta'sir ko'rsatadi, ammo boshqa o'smalarga emas, shuning uchun o'ziga xoslik va sezgirlikni namoyish etadi. Antikor shuningdek, nevus hujayralariga emas, balki intradermal nevuslarga va homila melanotsitlariga, ammo normal kattalar melanotsitlariga qarshi ijobiy ta'sir ko'rsatadi.
HMB-45 melanogenezning dalillarini ko'rsatadigan noyob o'smalar (masalan, pigmentli shvanoma, aniq hujayra sarkomasi) yoki tuberoz skleroz majmuasi (angiomiyolipoma va limfangiyomiya) bilan bog'liq bo'lgan o'smalar bundan mustasno, deyarli barcha melanoma bo'lmagan odamlarda yuzaga keladigan xavfli o'smalar bilan reaktiv emas.
Oldini olish
Voyaga etganlarning malign melanomani tekshirishini qo'llab-quvvatlovchi yoki rad etadigan dalillar yo'q.[86]
Ultraviyole nurlanish
Ultraviyole nurlanish manbalari (quyosh va quyosh to'shaklari) ta'sirini minimallashtirish,[87] quyoshdan himoya qilish choralariga rioya qilish va kiyish quyoshdan saqlovchi kiyim (uzun ko'ylaklar, uzun shimlar va keng bosh kiyimlar) himoya qilishni taklif qilishi mumkin.
Ko'nchilik uchun sun'iy nurdan foydalanish bir vaqtlar terining saraton kasalligini oldini olishga yordam beradi, deb ishonishgan, ammo bu aslida melanomalar bilan kasallanishning ko'payishiga olib kelishi mumkin.[88]
Lakni quritish uchun tirnoq salonlarida ishlatiladigan UV tirnoq lampalari, oldini olish mumkin bo'lgan yana bir keng tarqalgan va keng tarqalgan UV nurlanish manbai.[89][90] UV tirnoqli chiroq yordamida teri saratonini rivojlanish xavfi past bo'lishiga qaramay, UV tirnoqli chiroqni ishlatishdan oldin, qo'llaringizga barmoqsiz qo'lqop kiyish va / yoki SPF 30 yoki undan kattaroq quyoshdan saqlovchi krem surtish tavsiya etiladi.[89][90]
Tana hosil qilish uchun ultrabinafsha nurlaridan foydalanadi D vitamini shuning uchun D vitamini darajasini saqlab qolish va melanoma xavfini kamaytirish uchun etarli miqdorda quyosh nurlarini olishni muvozanatlash kerak; tanada bir kun davomida D vitamini hosil qilish uchun yarim soat quyosh nurlari kerak va bu adolatli teriga quyosh kuyishi uchun bir xil vaqtni oladi. Quyosh nurlari ta'sir qilish bir vaqtning o'zida emas, balki vaqti-vaqti bilan bo'lishi mumkin.[91]
Quyosh kremi
Quyosh kremi melanomani oldini olishda samarali ko'rinadi.[2][7] Ilgari, ochiq joylarda quyosh nurlaridan himoya qilish koeffitsienti (SPF) 50 va undan yuqori bo'lgan quyoshdan himoya qiluvchi kremlardan foydalanish tavsiya etilgan; chunki katta miqdordagi quyoshdan saqlovchi kremlar yuqori SPF bilan UVA ni samarali ravishda to'sib qo'yishadi.[92] Hozirda quyoshdan himoya qiluvchi yangi ingredientlar (avobenzon, rux oksidiva titanium dioksid) pastroq SPFlarda ham UVA va UVB ni samarali ravishda blokirovka qiladi. Quyoshdan himoya qiluvchi krem ham himoya qiladi skuamöz hujayrali karsinoma, boshqa teri saratoni.[93]
Quyoshdan himoya qiluvchi krem, quyoshning shikastlanishiga qarshi soxta xavfsizlik hissi yaratishi mumkin degan xavotirlar bildirildi.[94]
Dori vositalari
2005 yilgi tekshiruv shundan dalolat beradi statin va fibrat dorilar melanoma xavfini kamaytirishi mumkin.[95] Ammo 2006 yilgi sharh hech qanday foyda keltirmadi.[96]
Davolash
Klinik tashxisni tasdiqlash a bilan amalga oshiriladi teri biopsiyasi. Odatda bu chandiq yoki o'simtani kengroq qirqish bilan kuzatiladi. Sahnaga qarab, a qo'riqchi limfa tuguni biopsiya o'tkazilishi mumkin. Sentinel limfa tugunlari biopsiyasining sinov dalillari atrofida tortishuvlar mavjud;[97] 2015 yilga kelib foyda keltiradigan noaniq dalillar bilan.[98] Rivojlangan malign melanomani davolash multidisipliner yondashuvdan amalga oshiriladi.
Jarrohlik
Ekskizion biopsiya o'smani olib tashlashi mumkin, ammo takrorlanish xavfini kamaytirish uchun ko'pincha qo'shimcha operatsiya qilish kerak. Kerakli darajada to'liq jarrohlik eksizyon jarrohlik chekkalari va qisqa muddatli va uzoq muddatli kuzatuv bilan birga aniqlanadigan metastatik kasallik mavjudligini baholash standart hisoblanadi. Ko'pincha bu a tomonidan amalga oshiriladi keng mahalliy eksizyon (WLE) 1-2 sm (0,4-0,8 dyuym) chekkalari bilan. In situ Melanoma va lentigo malignlari torroq jarrohlik chekkalari bilan davolanadi, odatda 0,2-0,5 sm (0,1-0,2 dyuym). Ko'p jarrohlar 0,5 sm (0,2 dyuym) in-situ melanomani standart eksizyoni uchun parvarish standartini hisobga olishadi,[99] ammo chekka ostidagi operatsiya uchun 0,2 sm (0,1 dyuym) marj qabul qilinishi mumkin (Mohs operatsiyasi, yoki chekka nazorati bilan ikki yuzli texnika). Keng eksizyon asl zararlanish joyida o'smaning qaytalanish tezligini kamaytirishga qaratilgan. Bu melanomada davolanishning tez-tez uchraydigan usuli. So'nggi o'n yilliklarda kamroq tajovuzkor davolanishga bo'lgan umumiy tendentsiya bilan eksizyon uchun tegishli chegaralarni aniqlashga qaratilgan katta tadqiqotlar.[100] 2009 yildagi randomizatsiyalangan nazorat ostida o'tkazilgan tekshiruvlarning meta-tahlili, birlamchi teri melanomalarini keng eksizatsiyalashga yordam beradigan tirik qolish darajalarida kichik farqni aniqladi, ammo bu natijalar statistik jihatdan ahamiyatli emas edi.[101]
Mohs operatsiyasi 77% gacha davolanish darajasi bilan xabar qilingan[102] va in-situ melanoma uchun 98,0% gacha.[103] CCPDMA va "er-xotin skalpel" periferik chekka bilan boshqariladigan operatsiya ushbu "epiteliya" melanomasining samaradorligi bo'yicha Mohs operatsiyasiga tengdir.
Odatda tarqaladigan melanomalar buni shunday qiladi limfa tugunlari boshqa joyga tarqalmasdan oldin o'sma hududida. Limfa tugunlarini jarrohlik yo'li bilan olib tashlash orqali hayotni yaxshilashga urinishlar (limfadenektomiya) ko'plab asoratlar bilan bog'liq edi, ammo umuman omon qolish uchun foyda yo'q edi. Yaqinda qo'riqchi limfa tuguni limfa tugunlarida jarrohlik operatsiyasining asoratlarini kamaytirish uchun biopsiya ishlab chiqilgan, shu bilan birga tugunning o'sma bilan tutilishini baholash mumkin.[104]
Sentinel limfa tugunlarining biopsiyasi - bu teri melanomasini davolashda keng qo'llaniladigan protsedura.[105][106]
Erta, ingichka melanomani, shu jumladan in situ melanomani, T1a melanomani yoki T1b melanomani ≤ 0,5 mm baholash uchun na sentinel limfa tugunlari biopsiyasini, na boshqa diagnostik testlarni o'tkazmaslik kerak.[107] Bunday kasallikka chalingan odamlarning saraton kasalligi ularning limfa tugunlariga yoki boshqa joyga tarqalishi ehtimoldan yiroq emas va 5 yillik hayot darajasi 97% ni tashkil qiladi.[107] Ushbu fikrlar tufayli sentinel limfa tugunlarining biopsiyasi ko'rib chiqiladi keraksiz sog'liqni saqlash ular uchun.[107] Bundan tashqari, boshlang'ich qon tekshiruvlari va rentgenografik tadqiqotlar faqat ushbu turdagi melanomani aniqlash asosida amalga oshirilmasligi kerak, chunki saratonni aniqlash uchun aniqroq testlar mavjud va bu testlar yuqori ijobiy-ijobiy ko'rsatkichlarga ega.[107] Soxta ijobiy tomonlarni potentsial ravishda to'g'rilash uchun genlarni ekspressiyasini profilaktikasi noaniq va mayda shikastlanishlar uchun yordamchi sinov sifatida ishlatilishi mumkin.[108][109]
Sentinel limfa tugunlari biopsiyasi ko'pincha, ayniqsa T1b / T2 + o'smalari, shilliq qavat o'smalari, ko'z melanomasi va oyoq-qo'llar o'smalari uchun amalga oshiriladi.[iqtibos kerak] Jarayon deb nomlangan limfosintigrafiya kuzatuv tugunlari (larini) lokalizatsiya qilish uchun o'sma joyiga radioaktiv iz qoldiruvchi vosita amalga oshiriladi. Moviy iz qoldiruvchi yordamida qo'shimcha aniqlik beriladi bo'yoqva tugun (lar) ni biopsiya qilish uchun operatsiya o'tkaziladi. Muntazam gematoksilin va eozin (H&E) va immunoperoksidaza binoni tugun ishtirokini istisno qilish uchun etarli bo'ladi. Polimeraza zanjiri reaktsiyasi Odatda (PCR) tugunlarni sinovlari, odatda klinik tekshiruvlarga kirish uchun sinov uchun o'tkazilgan, hozirda salbiy sentinel limfa tuguniga ega bo'lgan ko'plab bemorlarning tugunlarida ozgina miqdorda musbat hujayralar bo'lganligi isbotlangan. Shu bilan bir qatorda, a ingichka igna aspiratsiyasi biopsiya o'tkazilishi mumkin va ko'pincha massalarni sinash uchun ishlatiladi.
Agar limfa tuguni ijobiy bo'lsa, limfa tugunining tarqalish darajasiga qarab, ko'pincha radikal limfa tugunini ajratish amalga oshiriladi. Agar kasallik to'liq rezektsiya qilingan bo'lsa, bemor yordamchi terapiya choralarini ko'radi teri biopsiyasi tanlovni boshqarishdir. Bu erda shubhali lezyon atrofdagi teri va to'qimalarning etarli (ammo minimal, odatda 1 yoki 2 mm) ellipsi bilan butunlay yo'q qilinadi.[110] Mahalliy limfa drenajining buzilishining oldini olish uchun dastlabki biopsiya uchun afzal qilingan jarrohlik chegarasi tor (1 mm) bo'lishi kerak. Biopsiya terining epidermal, dermal va teri osti qatlamlarini o'z ichiga olishi kerak. Bu esa histopatolog mikroskopik tekshiruv orqali melanoma qalinligini aniqlash. Bu tomonidan tasvirlangan Breslouning qalinligi (millimetr bilan o'lchanadi). Ammo katta zararlanishlar, masalan, lentigo malignaga shubha qilish yoki jarrohlik yo'li bilan qiyin bo'lgan joylar (yuz, oyoq barmoqlari, barmoqlar, ko'z qovoqlari) uchun vakili joylardagi kichik musht biopsiyasi etarli ma'lumot beradi va yakuniy sahnalashtirish yoki chuqurlikni aniqlashni buzmaydi. . Hech qanday holatda dastlabki biopsiya jarrohlikning so'nggi chegarasini (0,5 sm, 1,0 sm yoki 2 sm) o'z ichiga olmaydi, chunki noto'g'ri tashxis qo'yish ortiqcha chandiqlarga olib kelishi va kasallanish protseduradan. Katta boshlang'ich eksizyon mahalliy limfa drenajini buzadi va keyingi limfangiogramma bilan boshqariladigan limfnod diseksiyasiga ta'sir qilishi mumkin. Kichkina zarba biopsiyasidan har qanday vaqtda foydalanish mumkin, bu erda logistik va shaxsiy sabablarga ko'ra bemor ko'proq invaziv ekskizion biopsiyadan voz kechadi. Kichik zarbli biopsiya minimal invaziv va tezda davolanadi, odatda sezilarli iz qoldirmasdan.
Davolashga qo'shing
Yuqori xavfli melanomalar talab qilinishi mumkin yordamchi davolash, turli mamlakatlarda bunga munosabat turlicha bo'lishiga qaramay. In the United States, most patients in otherwise good health will begin up to a year of high-dose interferon treatment, which has severe side effects, but may improve the patient's prognosis slightly.[111] Biroq, Britaniya dermatologlari assotsiatsiyasi guidelines on melanoma state that interferon is not recommended as a standard adjuvant treatment for melanoma.[112] A 2013 meta-analysis suggested that the addition of interferon alpha increased disease-free and overall survival for people with AJCC TNM stage II-III cutaneous melanoma.[113] A 2011 meta-analysis showed that interferon could lengthen the time before a melanoma comes back but increased survival by only 3% at 5 years. The unpleasant side effects also greatly decrease quality of life.[114]
In Europe, interferon is usually not used outside the scope of clinical trials.[115][116]
Kimyoviy terapiya
Chemotherapy drugs such as Dacarbazine have been the backbone of metastatic melanoma treatment since FDA approval in 1975 however, its efficacy in terms of survival has never been proven in an RCT.[117]
In people with locally advanced cutaneous malignancies and sarcoma, isolated limb infusion (ILI) has been found to be a minimally invasive and well-tolerated procedure for delivering regional chemotherapy.[118][119]
Maqsadli terapiya
Melanoma cells have mutations that allow them to survive and grow indefinitely in the body.[117] Small-molecule targeted therapies work by blocking the genes involved in pathways for tumor proliferation and survival.[117] The main treatments are BRAF, C to'plami va NRAS inhibitörler.[120] These inhibitors work to inhibit the downstream pathways involved in cell proliferation and tumour development due to specific gene mutations.[121] People can be treated with small-molecule targeted inhibitors if they are positive for the specific mutation.[117] BRAF inhibitörleri, kabi vemurafenib va dabrafenib va a MEK inhibitori trametinib are the most effective, approved treatments for BRAF positive melanoma.[122][117] Melanoma tumors can develop qarshilik during therapy which can make therapy no longer effective, but combining the use of BRAF and MEK inhibitors may create a fast and lasting melanoma therapy response.[123]
A number of treatments improve survival over traditional chemotherapy.[117] Biochemotherapy (chemotherapy with cytokines IL-2 and IFN-α) combined with BRAF inhibitors improved survival for people with BRAF positive melanoma.[117] Biochemotherapy alone did not improve overall survival and had higher toxicity than chemotherapy.[117] Combining multiple chemotherapy agents (polychemotherapy) did not improve survival over monochemotherapy.[117] Targeted therapies result in relatively short progressiyasiz omon qolish (PFS) times. The therapy combination of dabrafenib and trametinib has a 3-year PFS of 23%, and 5-year PFS of 13%.[124]
Immunoterapiya
Immunoterapiya is aimed at stimulating the person's immune system against the tumor, by enhancing the body's own ability to recognize and kill cancer cells.[125] The current approach to treating melanoma with immunotherapy includes three broad categories of treatments including sitokinlar, immune check point inhibitors, and asrab oluvchi hujayralarni ko'chirish.[125] These treatment options are most often used in people with metastatic melanoma and significantly improves overall survival.[117] However, these treatments are often costly. For example, one immune check point inhibitor treatment, pembrolizumab, costs $10,000 to US$12,000 for a single dose administered every 3 weeks.[126]
Cytokine therapies used for melanoma include IFN-a va Il-2.[127] IL-2 (Proleukin) was the first new therapy approved (1990 Europe, 1992 USA) for the treatment of metastatic melanoma in 20 years.[iqtibos kerak] IL-2 may offer the possibility of a complete and long-lasting remission in this disease in a small percentage of people with melanoma.[128] Intralesional IL-2 for in-transit metastases has a high complete response rate ranging from 40 to 100%.[122] Xuddi shunday, IFN-a has shown only modest survival benefits and high toxicity, limiting its use as a stand-alone therapy.[117][127]
Immune check point inhibitors include anti-CTLA-4 monoclonal antibodies (ipilimumab va tremelimumab), pullik retseptorlari (TLR) agonists, CD40 agonists, anti-PD-1 (pembrolizumab, pidilizumabva nivolumab) va PD-L1 antikorlar.[125][127] Evidence suggests that anti-PD-1 antibodies are more effective than anti-CTLA4 antibodies with less systemic toxicity.[117] The five-year progression-free survival for immunotherapy with pembrolizumab is 21%.[124] A therapeutic approach that includes the combination of different therapies improves overall survival and progression-free survival compared to treatment with the separate immunotherapy drugs alone.[117]
Ongoing research is looking at treatment by asrab oluvchi hujayralarni ko'chirish.[129] Adoptive cell transfer refers to the application of pre-stimulated, modified T hujayralari yoki dendritik hujayralar and is presently used to minimize complications from laxta-qarshi xastalik.[127][130]
Lentigo maligna
Standard excision is still being done by most surgeons. Unfortunately, the recurrence rate is exceedingly high (up to 50%). This is due to the ill-defined visible surgical margin, and the facial location of the lesions (often forcing the surgeon to use a narrow surgical margin). The narrow surgical margin used, combined with the limitation of the standard "bread-loafing" technique of fixed tissue histology – result in a high "false negative" error rate, and frequent recurrences. Margin control (peripheral margins) is necessary to eliminate the false negative errors. Agar non noni is used, distances from sections should approach 0.1 mm to assure that the method approaches complete margin control. A meta-analysis of the literature in 2014 found no randomized controlled trials of surgical interventions to treat lentigo maligna or melanoma in-situ, even though surgery is the most widely used treatment.[131]
Mohs operatsiyasi has been done with cure rate reported to be as low as 77%,[102] and as high as 95% by another author.[103] The "double scalpel" peripheral margin controlled excision method approximates the Mohs method in margin control, but requires a pathologist intimately familiar with the complexity of managing the vertical margin on the thin peripheral sections and staining methods.[132]
Some melanocytic nevi, and melanoma-in-situ (lentigo maligna) have resolved with an experimental treatment, imiquimod (Aldara) topical cream, an immune enhancing agent. Some dermasurgeons are combining the 2 methods: surgically excising the cancer and then treating the area with Aldara cream postoperatively for three months. While some studies have suggested the adjuvant use of topical tazarotene, the current evidence is insufficient to recommend it and suggests that it increases topical inflammation, leading to lower patient compliance.[131]
Radiatsiya
Radiatsiya terapiyasi is often used after surgical resection for patients with locally or regionally advanced melanoma or for patients with unresectable distant metastases. Kilovoltage x-ray beams are often used for these treatments and have the property of the maximum radiation dose occurring close to the skin surface.[133] It may reduce the rate of local recurrence but does not prolong survival.[134] Radioimmunotherapy of metastatic melanoma is currently under investigation.Radiotherapy has a role in the palliation of metastatic melanoma.[135]
Prognoz
Factors that affect prognoz quyidagilarni o'z ichiga oladi:
- o'sma thickness in millimeters (Breslovning chuqurligi),
- depth related to skin structures (Clark level),
- type of melanoma,
- presence of ulceration,
- presence of lymphatic/perineural invasion,
- presence of tumor-infiltrating limfotsitlar (if present, prognosis is better),
- location of lesion,
- presence of satellite lesions, and
- presence of regional or distant metastaz.[136]
Certain types of melanoma have worse prognoses but this is explained by their thickness. Less invasive melanomas even with lymph node metastases carry a better prognosis than deep melanomas without regional metastasis at time of staging. Local recurrences tend to behave similarly to a primary unless they are at the site of a keng mahalliy eksizyon (as opposed to a staged excision or punch/shave excision) since these recurrences tend to indicate lymphatic invasion.
When melanomas have spread to the limfa tugunlari, one of the most important factors is the number of nodes with malignancy. Extent of malignancy within a node is also important; micrometastases in which malignancy is only microscopic have a more favorable prognosis than macrometastases. In some cases micrometastases may only be detected by special staining, and if malignancy is only detectable by a rarely employed test known as the polimeraza zanjiri reaktsiyasi (PCR), the prognosis is better. Macrometastases in which malignancy is clinically apparent (in some cases cancer completely replaces a node) have a far worse prognosis, and if nodes are matted or if there is extracapsular extension, the prognosis is worse still. In addition to these variables, expression levels and copy number variations of a number of relevant genes may be used to support assessment of malignant melanoma prognosis.[108][109]
Stage IV melanoma, in which it has metastasized, is the most deadly skin malignancy: five-year survival is 22.5%.[124] When there is distant metastasis, the cancer is generally considered incurable. The five-year survival rate is less than 10%.[137] The median survival is 6–12 months. Treatment is palliativ, focusing on life extension and hayot sifati. In some cases, patients may live many months or even years with metastatic melanoma (depending on the aggressiveness of the treatment). Metastases to skin and lungs have a better prognosis. Metastases to brain, bone and liver are associated with a worse prognosis. Survival is better with metastasis in which the location of the primary tumor is unknown.[138]
There is not enough definitive evidence to adequately stage, and thus give a prognosis for, ocular melanoma and melanoma of soft parts, or mucosal melanoma (e.g., rectal melanoma), although these tend to metastasize more easily. Even though regression may increase survival, when a melanoma has regressed, it is impossible to know its original size and thus the original tumor is often worse than a pathology report might indicate.
About 200 genes are prognostic in melanoma, with both unfavorable genes where high expression is correlated to poor survival and favorable genes where high expression is associated with longer survival times. Examples of unfavorable genes are MCM6 va TIMELESS; an example of a favorable gene is WIPI1.[45][46]
An increased neutorphil-to-lymphocyte ratio is associated with worse outcomes.[139]
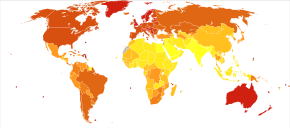
Epidemiologiya

Globally, in 2012, melanoma occurred in 232,000 people and resulted in 55,000 deaths.[2] Australia and New Zealand have the highest rates of melanoma in the world.[2] It has become more common in the last 20 years in areas that are mostly Kavkaz.[2]
The rate of melanoma has increased in the recent years, but it is not clear to what extent changes in behavior, in the environment, or in early detection are involved.[141]
Avstraliya
Avstraliya has a very high – and increasing – rate of melanoma. In 2012, deaths from melanoma occurred in 7.3–9.8 per 100,000 population. In Australia, melanoma is the third most common cancer in either sex; indeed, its incidence is higher than for o'pka saratoni, although the latter accounts for more deaths. It is estimated that in 2012, more than 12,000 Australians were diagnosed with melanoma: given Australia's modest population, this is better expressed as 59.6 new cases per 100,000 population per year; >1 in 10 of all new cancer cases were melanomas.[142] Melanoma incidence in Australia is matter of significance, for the following reasons:
- Australian melanoma incidence has increased by more than 30 per cent between 1991 and 2009.
- Australian melanoma age-standardised incidence rates were, as of 2008, at least 12 times higher than the world average.
- Australian melanoma incidence is, by some margin, the highest in the world.
- Overall age-standardised cancer incidence in Australia is the highest in the world, and this is attributable to melanoma alone. Age-standardised overall cancer incidence is similar to New Zealand, but there is a statistically-significant difference between Australia and all other parts of the developed world including North America, Western Europe, and the Mediterranean.
Qo'shma Shtatlar
In the United States about 9,000 people die from melanoma a year.[143] In 2011 it affected 19.7 per 100,000, and resulted in death in 2.7 per 100,000.[143]
2013 yilda:
- 71,943 people in the United States were diagnosed with melanomas of the skin, including 42,430 men and 29,513 women.
- 9,394 people in the United States died from melanomas of the skin, including 6,239 men and 3,155 women.[144]
The American Cancer Society's estimates for melanoma incidence in the United States for 2017 are:
- About 87,110 new melanomas will be diagnosed (about 52,170 in men and 34,940 in women).
- About 9,730 people are expected to die of melanoma (about 6,380 men and 3,350 women).
Melanoma is more than 20 times more common in whites than in African Americans. Overall, the lifetime risk of getting melanoma is about 2.5% (1 in 40) for whites, 0.1% (1 in 1,000) for African Americans, and 0.5% (1 in 200) for Hispanics.
The risk of melanoma increases as people age. The average age of people when the disease is diagnosed is 63.[145]
Tarix
Although melanoma is not a new disease, evidence for its occurrence in antiquity is rather scarce. However, one example lies in a 1960s examination of nine Peru mummies, radiokarbon dated to be approximately 2400 years old, which showed apparent signs of melanoma: melanotic masses in the skin and diffuse metastases to the bones.[146]
Jon Hunter is reported to be the first to operate on metastatic melanoma in 1787. Although not knowing precisely what it was, he described it as a "cancerous fungous excrescence". The excised tumor was preserved in the Ovchilar muzeyi ning Angliya qirollik jarrohlar kolleji. It was not until 1968 that microscopic examination of the specimen revealed it to be an example of metastatic melanoma.[147]
Frantsuz shifokori Rene Laennec was the first to describe melanoma as a disease entity. His report was initially presented during a lecture for the Faculté de Médecine de Paris in 1804 and then published as a bulletin in 1806.[148]
The first English-language report of melanoma was presented by an English general practitioner from Stourbridge, William Norris in 1820.[149] In his later work in 1857 he remarked that there is a familial predisposition for development of melanoma (Eight Cases of Melanoz with Pathological and Therapeutical Remarks on That Disease). Norris was also a pioneer in suggesting a link between nevi and melanoma and the possibility of a relationship between melanoma and environmental exposures, by observing that most of his patients had pale complexions.[150] He also described that melanomas could be amelanotic and later showed the metastatic nature of melanoma by observing that they can disseminate to other visceral organs.
The first formal acknowledgment of advanced melanoma as untreatable came from Samuel Kuper in 1840. He stated that the only chance for a cure depends upon the early removal of the disease (i.e., early excision of the malignant mole) ...'[151]
More than one and a half centuries later this situation remains largely unchanged.
Terminologiya
So'z melanoma came to English from 19th-century Yangi lotin[152] va foydalanadi shakllarni birlashtirish dan olingan qadimgi yunoncha ildizlari: melano- (belgilaydigan melanin) + -oma (denoting a tissue mass and especially a neoplazma), in turn from Yunoncha μέλας melalar, "dark",[153] va -ωμα oma, "process". So'z melanoma has a long history of being used in a broader sense to refer to any melanotsitik o'sma, typically, but not always malignant,[154][155] but today the narrower sense referring only to malignant types has become so dominant that benign tumors are usually not called melanomas anymore and the word melanoma is now usually taken to mean malignant melanoma unless otherwise specified. Terms such as "benign melanotsitik o'sma" unequivocally label the benign types, and modern histopathologic tumor classifications used in medicine do not use the word for benign tumors.
Tadqiqot
Pharmacotherapy research for unresectable or metastatic malignant melanoma is ongoing.[156]
Targeted therapies
In clinical research, adoptive cell therapy and gen terapiyasi, are being tested.[157]
Two kinds of experimental treatments developed at the Milliy saraton instituti (NCI), have been used in metastatic melanoma with tentative success.[36]
The first treatment involves adoptive cell therapy (ACT) using TILs immune cells (tumor-infiltrating lymphocytes) isolated from a person's own melanoma tumor.[122] These cells are grown in large numbers in a laboratory and returned to the patient after a treatment that temporarily reduces normal T cells in the patient's body. TIL therapy following lymphodepletion can result in durable complete response in a variety of setups.[158][159]
The second treatment, adoptive transfer of genetically altered autologous lymphocytes, depends on delivering genes that encode so called T hujayralari retseptorlari (TCRs), into patient's lymphocytes.[122] After that manipulation lymphocytes recognize and bind to certain molecules found on the surface of melanoma cells and kill them.[160]
A cancer vaccine showed modest benefit in late-stage testing in 2009 against melanoma.[161][162]
BRAF inhibitörleri
About 60% of melanomas contain a mutation in the B-Raf gene. Early clinical trials suggested that B-Raf inhibitors including Plexxicon's vemurafenib could lead to substantial tumor regression in a majority of patients if their tumor contain the B-Raf mutation.[163] In June 2011, a large klinik sinov confirmed the positive findings from those earlier trials.[164][165]
In August 2011 Vemurafenib received FDA approval for the treatment of late-stage melanoma. 2013 yil may oyida AQSh FDA approved dabrafenib as a single agent treatment for patients with BRAF V600E mutation-positive advanced melanoma.[166]
Some researchers believe that combination therapies that simultaneously block multiple pathways may improve efficacy by making it more difficult for the tumor cells to mutate before being destroyed. In October 2012 a study reported that combining Dabrafenib with a MEK inhibitori trametinib led to even better outcomes. Compared to Dabrafenib alone, progression-free survival was increased to 41% from 9%, and the median progressiyasiz omon qolish increased to 9.4 months versus 5.8 months. Some side effects were, however, increased in the combined study.[167][168]
In January 2014, the FDA approved the combination of dabrafenib and trametinib for the treatment of people with BRAF V600E/K-mutant metastatic melanoma.[169] In June 2018, the FDA approved the combination of a BRAF inhibitor encorafenib and a MEK inhibitor binimetinib for the treatment of unresectable or metastatic melanoma with a BRAF V600E or V600K mutation.[170]
Eventual resistance to BRAF and MEK inhibitors may be due to a cell surface protein known as EphA2 which is now being investigated.[171]
Ipilimumab
Da Amerika Klinik Onkologiya Jamiyati Conference in June 2010, the Bristol-Mayers Squibb pharmaceutical company reported the clinical findings of their drug ipilimumab. The study found an increase in median survival from 6.4 to 10 months in patients with advanced melanomas treated with the monoclonal ipilimumab, versus an experimental vaccine. It also found a one-year survival rate of 25% in the control group using the vaccine, 44% in the vaccine and ipilimumab group, and 46% in the group treated with ipilimumab alone.[172] However, some have raised concerns about this study for its use of the unconventional control arm, rather than comparing the drug against a placebo or standard treatment.[173][174] The criticism was that although Ipilimumab performed better than the vaccine, the vaccine has not been tested before and may be causing toxicity, making the drug appear better by comparison.
Ipilimumab was approved by the FDA in March 2011 to treat patients with late-stage melanoma that has spread or cannot be removed by surgery.[175][176][177]
In June 2011, a clinical trial of ipilimumab plus dakarbazin combined this immune system booster with the standard chemotherapy drug that targets cell division. It showed an increase in median survival for these late stage patients to 11 months instead of the 9 months normally seen. Researchers were also hopeful of improving the five year survival rate, though serious adverse side-effects were seen in some patients. A course of treatment costs $120,000. The drug's brandname is Yervoy.[164][178]
Surveillance methods
Advances in high resolution ultrasound scanning have enabled surveillance of metastatic burden to the sentinel lymph nodes.[179] The Screening and Surveillance of Ultrasound in Melanoma trial (SUNMEL) is evaluating ultrasound as an alternative to invasive surgical methods.[180]
Onkolitik viroterapiya
In some countries oncolytic virotherapy methods are studied and used to treat melanoma. Oncolytic virotherapy is a promising branch of viroterapiya, qayerda onkolitik viruslar are used to treat diseases; viruses can increase metabolism, reduce anti-tumor immunity and disorganize vasculature.[181] Talimogene laherparepvec (T-VEC) (which is a herpes simplex virus type 1–derived oncolytic immunotherapy), was shown to be useful against metastatic melanoma in 2015 with an increased survival of 4.4 months.[182][9]
Izohlar
Adabiyotlar
- ^ a b v d e f g h men j k l m "Melanoma Treatment – for health professionals". Milliy saraton instituti. 2015 yil 26 iyun. Arxivlandi asl nusxasidan 2015 yil 4 iyuldagi. Olingan 30 iyun 2015.
- ^ a b v d e f g h men j k l m n o p q r Dunyo bo'yicha saraton kasalligi to'g'risidagi hisobot (PDF). Jahon Sog'liqni saqlash tashkiloti. 2014. 5.14-bob. ISBN 978-9283204299. Arxivlandi (PDF) asl nusxasidan 2014-05-30.
- ^ Goldstein BG, Goldstein AO (April 2001). "Diagnosis and management of malignant melanoma". Amerika oilaviy shifokori. 63 (7): 1359–68, 1374. PMID 11310650.
- ^ a b "SEER Stat Fact Sheets: Melanoma of the Skin". NCI. Arxivlandi from the original on 2014-07-06.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Buta ZA, Braun A va boshq. (GBD 2015 Disease Injury Incidence Prevalence Collaborators) (October 2016). "1990-2015 yillarda 310 kasallik va jarohatlar bo'yicha global, mintaqaviy va milliy kasallik, tarqalish va nogironlik bilan yashagan: 2015 yilgi Global yuklarni o'rganish uchun tizimli tahlil". Lanset. 388 (10053): 1545–1602. doi:10.1016 / S0140-6736 (16) 31678-6. PMC 5055577. PMID 27733282.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Buta ZA, Braun A va boshq. (GBD 2015 Mortality Causes of Death Collaborators) (October 2016). "1980-2015 yillarda 249 ta o'limning global, mintaqaviy va milliy umr ko'rish davomiyligi, barcha sabablarga ko'ra o'lim va o'ziga xos o'lim: 2015 yilgi Global yuklarni o'rganish uchun tizimli tahlil". Lanset. 388 (10053): 1459–1544. doi:10.1016 / s0140-6736 (16) 31012-1. PMC 5388903. PMID 27733281.
- ^ a b Kanavy HE, Gerstenblith MR (2011 yil dekabr). "Ultraviyole nurlanish va melanoma". Seminars in Cutaneous Medicine and Surgery. 30 (4): 222–8. doi:10.1016 / j.sder.2011.08.003. PMID 22123420.
- ^ a b Azoury SC, Lange JR (oktyabr 2014). "Epidemiologiya, xavf omillari, profilaktika va melanomani erta aniqlash". Shimoliy Amerikaning jarrohlik klinikalari. 94 (5): vii, 945–62. doi:10.1016 / j.suc.2014.07.013. PMID 25245960.
- ^ a b Syn NL, Teng MW, Mok TS, Soo RA (2017 yil dekabr). "De-novo and acquired resistance to immune checkpoint targeting". Lanset. Onkologiya. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ^ "USCS Data Visualizations". gis.cdc.gov.
Need to select "melanoma"
- ^ "CDC - What Are the Symptoms of Skin Cancer?". www.cdc.gov. 2018-06-26. Olingan 2019-02-01.
- ^ Daniel Jensen J, Elewski BE (February 2015). "The ABCDEF Rule: Combining the "ABCDE Rule" and the "Ugly Duckling Sign" in an Effort to Improve Patient Self-Screening Examinations". The Journal of Clinical and Aesthetic Dermatology. 8 (2): 15. PMC 4345927. PMID 25741397.
- ^ "The EFG of Nodular Melanomas | MoleMap New Zealand". The EFG of Nodular Melanomas | MoleMap New Zealand. Olingan 2019-02-01.
- ^ Fiddler IJ (October 1995). "Melanoma Metastasis". Cancer Control. 2 (5): 398–404. doi:10.1177/107327489500200503. PMID 10862180.
- ^ a b v d e f "Melanoma Risk factors". Mayo klinikasi. Arxivlandi asl nusxasidan 2017-04-10. Olingan 2017-04-10.
- ^ a b Greene MH (December 1999). "The genetics of hereditary melanoma and nevi. 1998 update". Saraton. 86 (11 Suppl): 2464–77. doi:10.1002/(SICI)1097-0142(19991201)86:11+<2464::AID-CNCR3>3.0.CO;2-F. PMID 10630172.
- ^ a b v Goydos JS, Shoen SL (2016). "Acral Lentiginous Melanoma". Saraton kasalligini davolash va tadqiqotlar. 167: 321–9. doi:10.1007/978-3-319-22539-5_14. ISBN 978-3-319-22538-8. PMID 26601870.
- ^ Perkins A, Duffy RL (June 2015). "Atypical moles: diagnosis and management". Amerika oilaviy shifokori. 91 (11): 762–7. PMID 26034853.
- ^ Boniol M, Autier P, Boyle P, Gandini S (July 2012). "Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis". BMJ. 345: e4757. doi:10.1136/bmj.e4757. PMC 3404185. PMID 22833605.
- ^ El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. (WHO International Agency for Research on Cancer Monograph Working Group) (August 2009). "A review of human carcinogens--part D: radiation". Lanset. Onkologiya. 10 (8): 751–2. doi:10.1016/S1470-2045(09)70213-X. PMID 19655431.
- ^ Sanlorenzo M, Wehner MR, Linos E, Kornak J, Kainz W, Posch C, et al. (Yanvar 2015). "The risk of melanoma in airline pilots and cabin crew: a meta-analysis". JAMA Dermatologiya. 151 (1): 51–8. doi:10.1001/jamadermatol.2014.1077. PMC 4482339. PMID 25188246.
- ^ Rünger TM, Farahvash B, Hatvani Z, Rees A (January 2012). "Comparison of DNA damage responses following equimutagenic doses of UVA and UVB: a less effective cell cycle arrest with UVA may render UVA-induced pyrimidine dimers more mutagenic than UVB-induced ones". Fotokimyoviy va fotobiologik fanlar. 11 (1): 207–15. doi:10.1039/c1pp05232b. PMID 22005748.
- ^ Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS (May 2001). "Ultraviolet A and melanoma: a review". Amerika Dermatologiya Akademiyasining jurnali. 44 (5): 837–46. doi:10.1067/mjd.2001.114594. PMID 11312434. S2CID 7655216.
- ^ Oliveria SA, Saraiya M, Geller AC, Heneghan MK, Jorgensen C (February 2006). "Sun exposure and risk of melanoma". Bolalik davridagi kasalliklar arxivi. 91 (2): 131–8. doi:10.1136/adc.2005.086918. PMC 2082713. PMID 16326797.
- ^ Lee JA, Strickland D (May 1980). "Malignant melanoma: social status and outdoor work". Britaniya saraton jurnali. 41 (5): 757–63. doi:10.1038/bjc.1980.138. PMC 2010319. PMID 7426301.
- ^ Pion IA, Rigel DS, Garfinkel L, Silverman MK, Kopf AW (January 1995). "Occupation and the risk of malignant melanoma". Saraton. 75 (2 Suppl): 637–44. doi:10.1002/1097-0142(19950115)75:2+<637::aid-cncr2820751404>3.0.co;2-#. PMID 7804988.
- ^ "WHO | The World Health Organization recommends that no person under 18 should use a sunbed". JSSV. Arxivlandi asl nusxasi on June 16, 2009.
- ^ Khlat M, Vail A, Parkin M, Green A (May 1992). "Mortality from melanoma in migrants to Australia: variation by age at arrival and duration of stay". Amerika Epidemiologiya jurnali. 135 (10): 1103–13. doi:10.1093/oxfordjournals.aje.a116210. PMID 1632422.
- ^ Halachmi S, Gilchrest BA (March 2001). "Update on genetic events in the pathogenesis of melanoma". Onkologiyaning hozirgi fikri. 13 (2): 129–36. doi:10.1097/00001622-200103000-00008. PMID 11224711. S2CID 29876528.
- ^ "CDKN2A cyclin-dependent kinase inhibitor 2A (melanoma, p16, inhibits CDK4)". AQSh milliy tibbiyot kutubxonasi.
- ^ Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H (March 2016). "Hereditary melanoma: Update on syndromes and management: Genetics of familial atypical multiple mole melanoma syndrome". Amerika Dermatologiya Akademiyasining jurnali. 74 (3): 395–407, quiz 408–10. doi:10.1016/j.jaad.2015.08.038. PMC 4761105. PMID 26892650.
- ^ Firoz EF, Warycha M, Zakrzewski J, Pollens D, Wang G, Shapiro R, et al. (2009 yil aprel). "Association of MDM2 SNP309, age of onset, and gender in cutaneous melanoma". Klinik saraton tadqiqotlari. 15 (7): 2573–80. doi:10.1158/1078-0432.CCR-08-2678. PMC 3881546. PMID 19318491.
- ^ Bliss JM, Ford D, Swerdlow AJ, Armstrong BK, Cristofolini M, Elwood JM, et al. (1995 yil avgust). "Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. The International Melanoma Analysis Group (IMAGE)". Xalqaro saraton jurnali. 62 (4): 367–76. doi:10.1002/ijc.2910620402. PMID 7635560.
- ^ Miller AJ, Mihm MC (July 2006). "Melanoma". Nyu-England tibbiyot jurnali. 355 (1): 51–65. doi:10.1056/NEJMra052166. PMID 16822996.
- ^ Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MC, Sober AJ (December 1987). "Risk factors for cutaneous melanoma. A practical method of recognizing predisposed individuals". JAMA. 258 (21): 3146–54. doi:10.1001/jama.258.21.3146. PMID 3312689.
- ^ a b Hershkovitz L, Schachter J, Treves AJ, Besser MJ (2010). "Focus on adoptive T cell transfer trials in melanoma". Klinik va rivojlanish immunologiyasi. 2010: 260267. doi:10.1155/2010/260267. PMC 3018069. PMID 21234353.
- ^ "ASCO Annual Meeting Proceedings Part I. Abstract: Protective effect of a brisk tumor infiltrating lymphocyte infiltrate in melanoma: An EORTC melanoma group study". Klinik onkologiya jurnali. 25 (18S): 8519. 2007. doi:10.1200/jco.2007.25.18_suppl.8519. Arxivlandi asl nusxasidan 2011-07-25.
- ^ Davies MA, Samuels Y (October 2010). "Analysis of the genome to personalize therapy for melanoma". Onkogen. 29 (41): 5545–55. doi:10.1038/onc.2010.323. PMC 3169242. PMID 20697348.
- ^ Barrett JC (1991). "Mutagenesis and Carcinogenesis". Yilda Brugge J, Curran T, Harlow E, McCormick F (eds.). Odam saratonining kelib chiqishi. Cold Spring Harbor Press. ISBN 0-87969-404-1 - Internet arxivi orqali.
- ^ Sage E, Girard PM, Francesconi S (January 2012). "Unravelling UVA-induced mutagenesis". Fotokimyoviy va fotobiologik fanlar. 11 (1): 74–80. doi:10.1039/c1pp05219e. PMID 21901217. S2CID 45189513.
- ^ Budden T, Bowden NA (January 2013). "The role of altered nucleotide excision repair and UVB-induced DNA damage in melanomagenesis". Xalqaro molekulyar fanlar jurnali. 14 (1): 1132–51. doi:10.3390/ijms14011132. PMC 3565312. PMID 23303275.
- ^ Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. (2012 yil may). "Melanoma genome sequencing reveals frequent PREX2 mutations". Tabiat. 485 (7399): 502–6. Bibcode:2012 yil natur.485..502B. doi:10.1038/nature11071. PMC 3367798. PMID 22622578.
- ^ Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, Shannon PT, et al. (2010 yil aprel). "Analysis of genetic inheritance in a family quartet by whole-genome sequencing". Ilm-fan. 328 (5978): 636–9. Bibcode:2010Sci ... 328..636R. doi:10.1126 / science.1186802. PMC 3037280. PMID 20220176.
- ^ Campbell CD, Chong JX, Malig M, Ko A, Dumont BL, Han L, et al. (2012 yil noyabr). "Ta'sischi populyatsiyada autozigotlik yordamida odamning mutatsion darajasini baholash". Tabiat genetikasi. 44 (11): 1277–81. doi:10.1038 / ng.2418. PMC 3483378. PMID 23001126.
- ^ a b "The human pathology proteome in melanoma – The Human Protein Atlas". www.proteinatlas.org. Olingan 2017-10-02.
- ^ a b Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. (2017 yil avgust). "A pathology atlas of the human cancer transcriptome". Ilm-fan. 357 (6352): eaan2507. doi:10.1126/science.aan2507. PMID 28818916. S2CID 206659235.
- ^ Ellrott K, Beyli MH, Saksena G, Kovington KR, Kandot C, Styuart S va boshq. (Mart 2018). "Ko'p genomli quvurlar yordamida o'simta hosilalarini mutatsiyaga chaqirish bo'yicha miqyosli ochiq ilmiy yondashuv". Hujayra tizimlari. 6 (3): 271-281.e7. doi:10.1016 / j.cels.2018.03.002. PMC 6075717. PMID 29596782.
- ^ Leslie MC, Bar-Eli M (January 2005). "Regulation of gene expression in melanoma: new approaches for treatment". Uyali biokimyo jurnali. 94 (1): 25–38. doi:10.1002/jcb.20296. PMID 15523674.
- ^ Bhoumik A, Singha N, O'Connell MJ, Ronai ZA (June 2008). "Regulation of TIP60 by ATF2 modulates ATM activation". Biologik kimyo jurnali. 283 (25): 17605–14. doi:10.1074/jbc.M802030200. PMC 2427333. PMID 18397884.
- ^ Bhoumik A, Jones N, Ronai Z (March 2004). "Transcriptional switch by activating transcription factor 2-derived peptide sensitizes melanoma cells to apoptosis and inhibits their tumorigenicity". Amerika Qo'shma Shtatlari Milliy Fanlar Akademiyasi materiallari. 101 (12): 4222–7. Bibcode:2004PNAS..101.4222B. doi:10.1073/pnas.0400195101. PMC 384722. PMID 15010535.
- ^ Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V (April 2008). "The role of ATF-2 in oncogenesis". BioEssays. 30 (4): 314–27. doi:10.1002/bies.20734. PMID 18348191.
- ^ Huang Y, Minigh J, Miles S, Niles RM (February 2008). "Retinoic acid decreases ATF-2 phosphorylation and sensitizes melanoma cells to taxol-mediated growth inhibition". Molekulyar signalizatsiya jurnali. 3: 3. doi:10.1186/1750-2187-3-3. PMC 2265711. PMID 18269766.
- ^ Parmiani G (March 2016). "Melanoma Cancer Stem Cells: Markers and Functions". Saraton. 8 (3): 34. doi:10.3390/cancers8030034. PMC 4810118. PMID 26978405.
- ^ a b Akbani R, Akdemir KC, Aksoy BA, Albert M, Ally A, Amin SB, et al. (Cancer Genome Atlas Network) (June 2015). "Teri melanomasining genomik tasnifi". Hujayra. 161 (7): 1681–96. doi:10.1016/j.cell.2015.05.044. PMC 4580370. PMID 26091043.
- ^ Ascierto PA, Kirkwood JM, Grob JJ, Simeone E, Grimaldi AM, Maio M, et al. (2012 yil iyul). "The role of BRAF V600 mutation in melanoma". Journal of Translational Medicine. 10 (1): 85. doi:10.1186/1479-5876-10-85. PMC 3391993. PMID 22554099.
- ^ Botton T, Talevich E, Mishra VK, Zhang T, Shain AH, Berquet C, et al. (Oktyabr 2019). "Genetic Heterogeneity of BRAF Fusion Kinases in Melanoma Affects Drug Responses". Hujayra hisobotlari. 29 (3): 573–588.e7. doi:10.1016/j.celrep.2019.09.009. PMC 6939448. PMID 31618628.
- ^ McEvoy CR, Xu H, Smith K, Etemadmoghadam D, San Leong H, Choong DY, et al. (2019 yil may). "Profound MEK inhibitor response in a cutaneous melanoma harboring a GOLGA4-RAF1 fusion". Klinik tadqiqotlar jurnali. 129 (5): 1940–1945. doi:10.1172/JCI123089. PMC 6486352. PMID 30835257.
- ^ Wiesner T, He J, Yelensky R, Esteve-Puig R, Botton T, Yeh I, et al. (2014 yil may). "Kinase fusions are frequent in Spitz tumours and spitzoid melanomas". Tabiat aloqalari. 5 (1): 3116. doi:10.1038/ncomms4116. PMC 4084638. PMID 24445538.
- ^ Yeh I, Tee MK, Botton T, Shain AH, Sparatta AJ, Gagnon A, et al. (2016 yil noyabr). "NTRK3 kinase fusions in Spitz tumours". Patologiya jurnali. 240 (3): 282–290. doi:10.1002/path.4775. PMC 5071153. PMID 27477320.
- ^ Yeh I, Botton T, Talevich E, Shain AH, Sparatta AJ, de la Fouchardiere A, et al. (2015 yil may). "Activating MET kinase rearrangements in melanoma and Spitz tumours". Tabiat aloqalari. 6 (1): 7174. doi:10.1038/ncomms8174. PMID 26013381.
- ^ a b Wurm EM, Soyer HP (October 2010). "Scanning for melanoma". Avstraliyalik Preskriber (33): 150–55. doi:10.18773/austprescr.2010.070. Arxivlandi asl nusxasi on 2010-10-19.
- ^ "Prevention: ABCD's of Melanoma". American Melanoma Foundation. Arxivlandi asl nusxasi on 2003-04-23.
- ^ Friedman RJ, Rigel DS, Kopf AW (1985). "Early detection of malignant melanoma: the role of physician examination and self-examination of the skin". Ca. 35 (3): 130–51. doi:10.3322/canjclin.35.3.130. PMID 3921200.
- ^ Dinnes J, Deeks JJ, Grainge MJ, Chuchu N, Ferrante di Ruffano L, Matin RN, et al. (Dekabr 2018). "Visual inspection for diagnosing cutaneous melanoma in adults". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 12 (12): CD013194. doi:10.1002/14651858.CD013194. PMC 6492463. PMID 30521684.
- ^ Dinnes J, Deeks JJ, Chuchu N, Ferrante di Ruffano L, Matin RN, Thomson DR, et al. (Dekabr 2018). "Dermoscopy, with and without visual inspection, for diagnosing melanoma in adults". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 12: CD011902. doi:10.1002/14651858.CD011902.pub2. PMC 6517096. PMID 30521682.
- ^ Dinnes J, Deeks JJ, Saleh D, Chuchu N, Bayliss SE, Patel L va boshq. (Cochrane Skin Group) (2018 yil dekabr). "Kattalardagi teri melanomasini tashxislash uchun aks ettiruvchi konfokal mikroskopi". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 12: CD013190. doi:10.1002 / 14651858.CD013190. PMC 6492459. PMID 30521681.
- ^ a b Mascaro JM, Mascaro JM (1998 yil noyabr). "Dermatologning nevusga nisbatan pozitsiyasi:" yomon o'rdak "dan" qizil qalpoqcha "gacha bo'lgan ko'rinish"". Dermatologiya arxivi. 134 (11): 1484–5. doi:10.1001 / archderm.134.11.1484. PMID 9828892.
- ^ a b "Dermoskopiyaga kirish". DermNet Yangi Zelandiya. Arxivlandi asl nusxasidan 2009-05-07.
- ^ Montgomery BD, Sadler GM (yanvar 2009). "Pigmentli lezyonlarning perforator biopsiyasi xavfli bo'lishi mumkin". Kanadalik oilaviy shifokor. 55 (1): 24, muallifning javobi 24. PMC 2628830. PMID 19155361.
- ^ Luk PP, Vilain R, Crainic O, Makkarti SW, Tompson JF, Scolyer RA (avgust 2015). "O'simta hujayralari implantatsiyasini keltirib chiqaradigan melanomaning perforator biopsiyasi: melanotsitik o'smalar uchun qisman biopsiyalardan foydalanishning yana bir xavfi". Avstraliya dermatologiyasi jurnali. 56 (3): 227–31. doi:10.1111 / ajd.12333. PMID 25827527.
- ^ Lin SW, Kaye V, Goldfarb N, Rawal A, Warshaw E (iyul 2012). "Punchli biopsiyadan so'ng melanoma o'simtasini ekish". Dermatologik jarrohlik. 38 (7 Pt 1): 1083-5. doi:10.1111 / j.1524-4725.2012.02384.x. PMID 22471244. S2CID 3431248.
- ^ Martin RC, Scoggins CR, Ross MI, Reintgen DS, Noyes RD, Edvards MJ, McMasters KM (dekabr 2005). "Melanomaning kesma biopsiyasi zararli emasmi?". Amerika jarrohlik jurnali. 190 (6): 913–7. doi:10.1016 / j.amjsurg.2005.08.020. PMID 16307945.
- ^ Yamashita Y, Xashimoto I, Abe Y, Seike T, Okava K, Senzaki Y va boshq. (2014 yil mart). "Xavfli melanoma bilan kasallangan bemorlarning tirik qolish darajasiga biopsiya texnikasining ta'siri". Plastik jarrohlik arxivi. 41 (2): 122–5. doi:10.5999 / aps.2014.41.2.122. PMC 3961608. PMID 24665419.
- ^ Mills SE (mart 2002). "Neyroendokrin karsinomalarga urg'u beradigan bosh va bo'yinning neyroektodermal neoplazmalari". Zamonaviy patologiya. 15 (3): 264–78. doi:10.1038 / modpathol.3880522. PMID 11904342. S2CID 34498802.
- ^ a b 805-bet: Ferri, Fred (2019). Ferrining klinik maslahatchisi 2019 yil: 1 ta 5 ta kitob. Filadelfiya, Pensilvaniya: Elsevier. ISBN 978-0-323-52957-0. OCLC [//www.worldcat.org/oclc/1040695302 1040695302.
- ^ Xiong M, Charifa A, Chen CS (2020). "Saraton, Lentigo Maligna melanomasi". StatPearls, Milliy Biotexnologiya Axborot Markazi. PMID 29489150. Oxirgi yangilanish: 2019 yil 18-may.
- ^ Piliang MP (sentyabr 2009). "Acral Lentiginous Melanoma". Jarrohlik patologiyasi klinikalari. 2 (3): 535–41. doi:10.1016 / j.path.2009.08.005. PMID 26838538.
- ^ McKenna JK, Florell SR, Goldman GD, Bowen GM (2006 yil aprel). "Lentigo maligna / lentigo maligna melanoma: diagnostika va davolashning hozirgi holati". Dermatologik jarrohlik. 32 (4): 493–504. doi:10.1111 / j.1524-4725.2006.32102.x. PMID 16681656. S2CID 8312676.
- ^ "Terining saraton kasalligi". Kanada saraton kasalligi jamiyati. Olingan 2020-02-26.
- ^ Fleming, C. (2010). "Lentigo maligna bilan og'rigan bemorlarni qanday boshqarish kerak". Melanoma tadqiqotlari. 20: e26. doi:10.1097 / 01.cmr.0000382797.99333.66. ISSN 0960-8931. S2CID 71464540.
- ^ Xeyl CS. "Terining melanotsitik o'smasi - Melanoma - Melanoma in situ". patologiya. Mavzu tugallandi: 2013 yil 1-may. Qayta ko'rib chiqilgan: 2019 yil 23-may
- ^ Park HS, Cho KH (aprel 2010). "In situ Acral lentiginous melanoma: diagnostika va boshqarish muammosi". Saraton. 2 (2): 642–52. doi:10.3390 / saraton kasalligi2020642. PMC 3835096. PMID 24281086.
- ^ Og'irligi RM, Viator JA, Deyl PS, Kolduell CW, Lisle AE (2006 yil oktyabr). "Inson qon aylanish tizimidagi metastatik melanoma hujayralarini fotoakustik aniqlash". Optik xatlar. 31 (20): 2998–3000. Bibcode:2006 yil OpTL ... 31.2998W. doi:10.1364 / OL.31.002998. PMID 17001379.
- ^ Dinnes J, Ferrante di Ruffano L, Takwoingi Y, Cheung ST, Natan P, Matin RN va boshq. (Cochrane Skin Group) (2019 yil iyul). "Teri melanomasi bo'lgan kattalarni sahnalashtirish va qayta sahnalashtirish uchun ultratovush, KT, MRI yoki PET-KT". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 7: CD012806. doi:10.1002 / 14651858.CD012806.pub2. PMC 6601698. PMID 31260100.
- ^ a b v d e Gershenvald JE, Scolyer RA, Gess KR, Sondak VK, Long GV, Ross MI va boshq. (2017 yil noyabr). "Melanomani sahnalashtirish: Saraton kasalligining sakkizinchi nashri bo'yicha Amerika Qo'shma Qo'mitasida dalillarga asoslangan o'zgarishlar". Ca. 67 (6): 472–492. doi:10.3322 / caac.21409. PMC 5978683. PMID 29028110.iqtibos keltirgan holda
Amin MB, Edge SB, Greene FL va boshq. AJCC saraton kasalligini boshqarish bo'yicha qo'llanma. 8-nashr. Nyu-York: Springer International Publishing; 2017: 563‐585). - ^ Yoxansson M, Brodersen J, Gotszhe Kompyuter, Yorgensen KJ va boshq. (Cochrane Skin Group) (iyun 2019). "Xatarli melanomada kasallik va o'limni kamaytirish bo'yicha skrining". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 6: CD012352. doi:10.1002 / 14651858.CD012352.pub2. PMC 6545529. PMID 31157404.
- ^ Autier P (oktyabr 2005). "Teri malign melanomasi: quyosh to'shaklari va quyoshdan saqlovchi vositalar to'g'risida ma'lumotlar". Saratonga qarshi terapiyani ekspertizasi. 5 (5): 821–33. doi:10.1586/14737140.5.5.821. PMID 16221052. S2CID 6091309.
- ^ Klou-Gorr KM, Titus-Ernstoff L, Perri AE, Spenser SK, Ernstoff MS (sentyabr 2008). "Quyosh nurlari, bronzalar va melanoma xavfi". Saraton kasalligi sabablari va nazorati. 19 (7): 659–69. doi:10.1007 / s10552-008-9129-6. PMC 6367654. PMID 18273687.
- ^ a b Shihab N, Lim HW (2018 yil noyabr). "UV tirnoq chiroqqa ta'sir etishi mumkin bo'lgan teri kanserogen xavfi: ko'rib chiqish". Fotodermatologiya, fotoimmunologiya va fotomeditsina. 34 (6): 362–365. doi:10.1111 / phpp.12398. PMID 29882991. S2CID 46958616.
- ^ a b O'Sullivan NA, Tait CP (may, 2014). "Solar to'shagi va tirnoq lampalaridan foydalanish va teri malignite xavfi: adabiyotlarni ko'rib chiqish". Avstraliya dermatologiyasi jurnali. 55 (2): 99–106. doi:10.1111 / ajd.12145. PMID 24592921.
- ^ Greinert R, de Vriz E, Erdmann F, Espina C, Auvinen A, Kesminiene A, Schüz J (dekabr 2015). "Saratonga qarshi Evropa kodeksi 4-nashr: Ultraviyole nurlanish va saraton". Saraton epidemiologiyasi. 39 Qo'shimcha 1 (Qo'shimcha 1): S75-83. doi:10.1016 / j.canep.2014.12.014. PMID 26096748.
- ^ "Melanomani oldini olish mumkinmi?". Arxivlandi asl nusxasi 2006 yil 27 iyunda.
- ^ Burnett ME, Vang SQ (aprel 2011). "Quyoshdan himoya qiluvchi dolzarb muammolar: tanqidiy sharh". Fotodermatologiya, fotoimmunologiya va fotomeditsina. 27 (2): 58–67. doi:10.1111 / j.1600-0781.2011.00557.x. PMID 21392107.
- ^ Planta MB (2011-11-01). "Quyoshdan himoya qiluvchi krem va melanoma: bizning profilaktika xabarimiz to'g'rimi?". Amerika oilaviy tibbiyot kengashi jurnali. 24 (6): 735–9. doi:10.3122 / jabfm.2011.06.100178. PMID 22086817. S2CID 1477485.
- ^ Dellavalle RP, Drake A, Graber M, Heilig LF, Hester EJ, Jonson KR va boshq. (2005 yil oktyabr). "Melanomani oldini olish uchun statinlar va fibratlar". Tizimli sharhlarning Cochrane ma'lumotlar bazasi (4): CD003697. doi:10.1002 / 14651858.CD003697.pub2. PMID 16235336.
- ^ Freeman SR, Drake AL, Heilig LF, Graber M, McNealy K, Schilling LM, Dellavalle RP (2006 yil noyabr). "Statinlar, fibratlar va melanoma xavfi: tizimli tahlil va meta-tahlil". Milliy saraton instituti jurnali. 98 (21): 1538–46. doi:10.1093 / jnci / djj412. PMID 17077356.
- ^ "Melanomadagi Sentinel tugunidagi biopsiya protsedurasi omon qolish uchun afzallik bermaydi". Xatarli melanoma. 2008-01-08. Arxivlandi asl nusxasi 2012-07-11. Olingan 2012-08-13.
- ^ Kyrgidis A, Tzellos T, Mocellin S, Apalla Z, Lallas A, Pilati P, Stratigos A (may 2015). "Sentinel limfa tugunlari biopsiyasi, so'ngra mahalliy teri melanomasi uchun limfa tugunlarini ajratish". Tizimli sharhlarning Cochrane ma'lumotlar bazasi (5): CD010307. doi:10.1002 / 14651858.CD010307.pub2. PMC 6461196. PMID 25978975.
- ^ Klark GS, Pappas-Politis EC, Cherpelis BS, Messina JL, Möller MG, Cruse CW, Glass LF (iyul 2008). "Quyoshdan doimiy zarar ko'rgan terida melanomani in situ jarrohlik yo'li bilan boshqarish". Saraton kasalligini nazorat qilish. 15 (3): 216–24. doi:10.1177/107327480801500304. PMID 18596673.
- ^ Balch CM, Urist MM, Karakousis CP, Smit TJ, Temple WJ, Drzewiecki K va boshq. (1993 yil sentyabr). "O'rta qalinlikdagi melanomalar (1 dan 4 mm gacha) uchun 2 sm jarrohlik chekkalarining samaradorligi. Ko'p muassasa randomizatsiyalangan jarrohlik tekshiruv natijalari". Jarrohlik yilnomalari. 218 (3): 262-7, munozara 267-9. doi:10.1097/00000658-199309000-00005. PMC 1242959. PMID 8373269.
- ^ Sladden MJ, Balch C, Barzilai DA, Berg D, Freiman A, Handiside T va boshq. (Oktyabr 2009). "Birlamchi teri melanomasi uchun jarrohlik eksizyon chegaralari". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 129 (4): CD004835. doi:10.1002 / 14651858.CD004835.pub2. PMID 19821334.
- ^ a b Mohs FE, Mixail GR (1991 yil yanvar). Mohs mikrografik jarrohligi. V.B. Saunders. 13-14 betlar. ISBN 978-0-7216-3415-9. Arxivlandi asl nusxasidan 2016-01-07.
- ^ a b Bene NI, Healy C, Coldiron BM (may 2008). "Mohs mikrografik jarrohligi melanoma in situ uchun 95,1% vaqtni to'g'ri bajaradi: 167 holatni istiqbolli o'rganish". Dermatologik jarrohlik. 34 (5): 660–4. doi:10.1111 / j.1524-4725.2007.34124.x. PMID 18261099. S2CID 23386371.
Kichkina melanoma in situ-da davolanish darajasi 98% ni tashkil qiladi va melanona in situ lentigo maligna variantida qayd etilgan 95% Mohs operatsiyasi.
- ^ "Melanoma tekshiruvida ultratovush tekshiruvi va nazorati (SUNMEL)". Arxivlandi asl nusxasi 2009-01-06 da.
- ^ Crowson AN, Haskell H (oktyabr 2013). "Teri melanomasini boshqarishda qo'riqchi limfa-tugun biopsiyasining ahamiyati". Giornale Italiano di Dermatologia va Venereologia. 148 (5): 493–9. PMID 24005142.
- ^ Ross MI, Gershenvald JE (2013 yil may-iyun). "Melanoma uchun limfa tugunlari biopsiyasi: yigirma yillik tajribadan so'ng dermatologlar uchun juda muhim yangilanish". Dermatologiya klinikalari. 31 (3): 298–310. doi:10.1016 / j.clindermatol.2012.08.004. PMID 23608449.
- ^ a b v d Amerika Dermatologiya Akademiyasi (2013 yil fevral), "Shifokorlar va bemorlar so'rashlari kerak bo'lgan beshta narsa", Aql bilan tanlash: ning tashabbusi ABIM Foundation, Amerika Dermatologiya Akademiyasi, dan arxivlangan asl nusxasi 2013 yil 1-dekabrda, olingan 5 dekabr 2013quyidagilarga ishora qiladi:
- Bichakjian CK, Halpern AC, Jonson TM, Foote Hood A, Grichnik JM, Swetter SM va boshq. (Amerika Dermatologiya Akademiyasi) (2011 yil noyabr). "Birlamchi teri melanomasini davolash bo'yicha ko'rsatmalar. Amerika Dermatologiya Akademiyasi". Amerika Dermatologiya Akademiyasining jurnali. 65 (5): 1032–47. doi:10.1016 / j.jaad.2011.04.031. PMID 21868127.
- Amerika saraton kasalligi bo'yicha qo'shma qo'mitasi (2010). Edge SB (tahrir). AJCC saratonini davolash bo'yicha qo'llanma (7-nashr). Springer. ISBN 978-0-387-88440-0.
- Milliy keng qamrovli saraton tarmog'i (2012), Onkologiya bo'yicha milliy keng qamrovli saraton kasalligining klinik qo'llanmasi (NCCN qo'llanmasi): melanoma (PDF), Vashington Fort, Pensilvaniya: Milliy keng qamrovli saraton tarmog'i, arxivlangan asl nusxasi (PDF) 2013 yil 28 dekabrda, olingan 5 dekabr 2013

- ^ a b van Kempen LC, Redpath M, Robert C, Spatz A (noyabr 2014). "Teri melanomasining molekulyar patologiyasi". Melanomani boshqarish. 1 (2): 151–164. doi:10.2217 / mmt.14.23. PMC 6094595. PMID 30190820.
- ^ a b Brunner G, Reitz M, Heinecke A, Lippold A, Berking C, Suter L, Atzpodien J (Fevral 2013). "Teri melanomasida klinik natijalarni bashorat qiluvchi to'qqizta gen imzosi". Saraton tadqiqotlari va klinik onkologiya jurnali. 139 (2): 249–58. doi:10.1007 / s00432-012-1322-z. PMID 23052696. S2CID 20257709.
- ^ Swanson NA, Li KK, Gorman A, Li HN (oktyabr 2002). "Biopsiya texnikasi. Melanoma diagnostikasi". Dermatologik klinikalar. 20 (4): 677–80. doi:10.1016 / S0733-8635 (02) 00025-6. PMID 12380054.
- ^ Kirkvud JM, Strawderman MH, Ernstoff MS, Smit TJ, Borden EC, Blum RH (yanvar 1996). "Yuqori darajadagi rezektsiya qilingan teri melanomasining interferon alfa-2b yordamchi terapiyasi: Sharqiy kooperativ onkologiya guruhi sinovi EST 1684". Klinik onkologiya jurnali. 14 (1): 7–17. doi:10.1200 / JCO.1996.14.1.7. PMID 8558223.
- ^ Cox NH, inglizcha JS, nashr. (2010). Britaniya dermatologlar assotsiatsiyasini boshqarish bo'yicha ko'rsatmalar. Villi-Blekvell. ISBN 978-1-4443-3552-1. Olingan 19 avgust 2013.
- ^ Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V (iyun 2013). "Teri melanomasini yordamchi davolash uchun interferon alfa". Tizimli sharhlarning Cochrane ma'lumotlar bazasi (6): CD008955. doi:10.1002 / 14651858.CD008955.pub2. PMID 23775773.
- ^ Wheatley K, Ives N, Eggermont A, Kirkwood J, Cascinelli N, Markovic SN, Hancock B, Lee S, Suciu S (2007). "Interferon-a melanoma uchun yordamchi terapiya sifatida: individual bemorning randomizatsiyalangan meta-tahlili". J Clin Oncol. 25 (18 ta qo'shimcha): 8526. doi:10.1200 / jco.2007.25.18_suppl.8526.
- ^ Kirkwood JM, Ibrohim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS va boshq. (Iyun 2000). "Xavfli melanomada yuqori va past dozali alfa-2b interferon: E1690 / S9111 / C9190 guruhlararo sinovining birinchi tahlili". Klinik onkologiya jurnali. 18 (12): 2444–58. doi:10.1200 / JCO.2000.18.12.2444. PMID 10856105.
- ^ Kirkvud JM, Ibrohim JG, Sondak VK, Ernstoff MS, Ross M (mart 2002). "Melanoma metastazlari uchun interferon alfa-2a". Lanset. 359 (9310): 978–9. doi:10.1016 / S0140-6736 (02) 08001-7. PMID 11918944. S2CID 33866115.
- ^ a b v d e f g h men j k l m Pasquali S, Xadjinikolau AV, Chiarion Sileni V, Rossi CR, Mocellin S (2018 yil fevral). "Metastatik teri melanomasini tizimli davolash usullari". Tizimli sharhlarning Cochrane ma'lumotlar bazasi. 2: CD011123. doi:10.1002 / 14651858.CD011123.pub2. PMC 6491081. PMID 29405038.
- ^ O'Donoghue C, Perez MC, Mullinax JE, Hardman D, Sileno S, Naqvi SM va boshq. (Dekabr 2017). "Izolyatsiya qilingan oyoq-qo'l infuzioni: 200 dan ortiq infuziya bilan yagona markaz tajribasi". Jarrohlik onkologiyasi yilnomalari. 24 (13): 3842–3849. doi:10.1245 / s10434-017-6107-9. PMID 29019175. S2CID 28907006.
- ^ Giles MH, Koventri BJ (2013 yil avgust). "Melanoma uchun izolyatsiyalangan oyoq-qo'l infuzion kimyoviy terapiyasi: Adelaida melanomasi bo'limidagi dastlabki tajribaga umumiy nuqtai". Saraton kasalligini boshqarish va tadqiqotlar. 5: 243–9. doi:10.2147 / cmar.s45746. PMC 3753062. PMID 23990731.
- ^ Berger M, Richtig G, Kashofer K, Aigelsreiter A, Richtig E (iyun 2018). "BRAFwt / NRASwt / KITwt melanomasida maqsadli terapiya uchun imkoniyatlar oynasi: termoyadroviy oqsillari va boshqa mutatsiyalar biologiyasi va klinik oqibatlari". Giornale Italiano di Dermatologia va Venereologia. 153 (3): 349–360. doi:10.23736 / S0392-0488.18.05970-9. PMID 29600692.
- ^ Broussard L, Howland A, Ryu S, Song K, Norris D, Armstrong CA, Song PI (sentyabr 2018). "Melanoma hujayralari o'limining mexanizmlari". Chonnam Medical Journal. 54 (3): 135–142. doi:10.4068 / cmj.2018.54.3.135. PMC 6165917. PMID 30288368.
- ^ a b v d Maverakis E, Kornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S va boshq. (2015 yil may). "Metastatik melanoma - davolashning amaldagi va kelajakdagi variantlarini ko'rib chiqish". Acta Dermato-Venereologica. 95 (5): 516–24. doi:10.2340/00015555-2035. PMID 25520039.
- ^ Kuske M, Westphal D, Wehner R, Shmitz M, Beissert S, Praetorius C, Meier F (oktyabr 2018). "BRAF va MEK inhibitörlerinin immunomodulyatsion ta'siri: Melanoma terapiyasining ta'siri". Farmakologik tadqiqotlar. 136: 151–159. doi:10.1016 / j.phrs.2018.08.019. PMID 30145328. S2CID 207369519.
- ^ a b v Rebekka, Vito V.; Herlin, Meenxard (2020). "Melanomada giyohvandlikka qarshilik ko'rsatishning nongenetik mexanizmlari". Saraton biologiyasining yillik sharhi. 4: 315–330. doi:10.1146 / annurev-cancerbio-030419-033533.
- ^ a b v Sanlorenzo M, Vujic I, Posch C, Dajee A, Yen A, Kim S va boshq. (Iyun 2014). "Melanoma immunoterapiyasi". Saraton biologiyasi va terapiyasi. 15 (6): 665–74. doi:10.4161 / cbt.28555. PMC 4049781. PMID 24651672.
- ^ Dranitsaris G, Zhu X, Adunlin G, Vinsent MD (avgust 2018). "Saratonga qarshi immuno-onkologik dorilar davrida iqtisodiy samaradorlik va arzonlik". Farmakoekonomika va natijalarni tadqiq qilish bo'yicha ekspert sharhi. 18 (4): 351–357. doi:10.1080/14737167.2018.1467270. PMID 29681201. S2CID 5079628.
- ^ a b v d G'arbiy HJ (aprel 2015). "JAMA Onkologiya kasallari sahifasi. Immunitetni nazorat qilish inhibitörleri". JAMA Onkologiya. 1 (1): 115. doi:10.1001 / jamaoncol.2015.0137. PMID 26182315.
- ^ Buzaid AC (2004 yil oktyabr). "Metastatik teri melanomasini boshqarish". Onkologiya. 18 (11): 1443-50, munozara 1457-9. PMID 15609471.
- ^ Rosenberg SA, Restifo NP (2015 yil aprel). "Odam saratoni uchun shaxsiy immunoterapiya sifatida qabul qiluvchi hujayralarni ko'chirish". Ilm-fan. 348 (6230): 62–8. Bibcode:2015 yil ... 348 ... 62R. doi:10.1126 / science.aaa4967. PMC 6295668. PMID 25838374.
- ^ Zang YW, Gu XD, Xiang JB, Chen ZY (iyun 2014). "Qattiq o'smalarda asrab oluvchi T hujayralari terapiyasining klinik qo'llanilishi". Tibbiyot fanlari monitori. 20: 953–9. doi:10.12659 / msm.890496. PMC 4063985. PMID 24912947.
- ^ a b Tzellos T, Kyrgidis A, Mocellin S, Chan AW, Pilati P, Apalla Z (dekabr 2014). "In situ melanoma uchun aralashuvlar, shu jumladan lentigo maligna". Tizimli sharhlarning Cochrane ma'lumotlar bazasi (12): CD010308. doi:10.1002 / 14651858.CD010308.pub2. PMID 25526608.
- ^ Jonson TM, Headington JT, Beyker SR, Lou L (noyabr 1997). "Lentigo maligna va lentigo maligna melanoma uchun bosqichma-bosqich eksizyonning foydaliligi:" kvadrat "protsedura". Amerika Dermatologiya Akademiyasining jurnali. 37 (5 Pt 1): 758-64. doi:10.1016 / S0190-9622 (97) 70114-2. PMID 9366823.
- ^ Hill R, Healy B, Holloway L, Kuncic Z, Twaites D, Baldock C (mart 2014). "Kilovoltaj rentgen nurlari dozimetriyasidagi yutuqlar". Tibbiyot va biologiyada fizika. 59 (6): R183-231. Bibcode:2014 PMB .... 59R.183H. doi:10.1088 / 0031-9155 / 59/6 / r183. PMID 24584183. S2CID 18082594.
- ^ Bastiaannet E, Beukema JK, Hoekstra HJ (2005 yil fevral). "Melanoma bilan kasallangan bemorlarda limfa tugunlari disektsiyasidan so'ng radiatsiya terapiyasi: davolash, natijasi va asoratlari". Saraton kasalligini davolash bo'yicha sharhlar. 31 (1): 18–26. doi:10.1016 / j.ctrv.2004.09.005. PMID 15707701.
- ^ Ford MB, Mitchell MF, Boyer KL, Ford MB, Judkins AF, Levin B (1999). "Saraton epidemiologiyasi". Birlamchi tibbiy yordam onkologiyasi. 1-27 betlar.
- ^ Homsi J, Kashani-Sabet M, Messina JL, Daud A (oktyabr 2005). "Teri melanomasi: prognostik omillar" (PDF). Saraton kasalligini nazorat qilish. 12 (4): 223–9. doi:10.1177/107327480501200403. PMID 16258493. Arxivlandi asl nusxasi (PDF) 2008-02-16.
- ^ Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG va boshq. (2001 yil avgust). "Teri melanomasi uchun saraton kasalligini boshqarish bo'yicha Amerika Qo'shma Qo'mitasining yakuniy versiyasi". Klinik onkologiya jurnali. 19 (16): 3635–48. CiteSeerX 10.1.1.475.6560. doi:10.1200 / JCO.2001.19.16.3635. PMID 11504745. Arxivlandi asl nusxasi 2006-03-05 da. Olingan 2006-07-31.
- ^ Bae JM, Choi YY, Kim DS, Li JH, Jang HS, Li JH va boshq. (Yanvar 2015). "Birlamchi noma'lum bo'lgan metastatik melanomalar prognozni ma'lum birlamchi ko'rsatkichlarga qaraganda yaxshiroq ko'rsatadi: kuzatuv ishlarini tizimli qayta ko'rib chiqish va meta-tahlil". Amerika Dermatologiya Akademiyasining jurnali. 72 (1): 59–70. doi:10.1016 / j.jaad.2014.09.029. PMID 25440435.
- ^ Zhan H, Ma JY, Jian QC (sentyabr 2018). "Melanoma bilan kasallangan bemorlarda neytrofil-limfotsitlar nisbatini oldindan davolashning prognostik ahamiyati: meta-tahlil". Clinica Chimica Acta; Xalqaro Klinik Kimyo jurnali. 484: 136–140. doi:10.1016 / j.cca.2018.05.055. PMID 29856976.
- ^ "CANCERMondial (GLOBOCAN)". GLOBOCAN. 2010. Arxivlandi asl nusxasidan 2012 yil 17 fevralda. Olingan 12 avgust 2010.
- ^ Bervik M, Viggins S (2006 yil may). "Teri malign melanomasining hozirgi epidemiologiyasi". Bioscience-dagi chegara. 11: 1244–54. doi:10.2741/1877. PMID 16368510.
- ^ "Avstraliyada saraton kasalligi: 2012 yil haqida umumiy ma'lumot". AIHW. Arxivlandi asl nusxasi 2014-06-02 da. Olingan 2014-06-01.
- ^ a b Guy GP, Tomas CC, Tompson T, Uotson M, Massetti GM, Richardson LC (iyun 2015). "Hayotiy belgilar: melanoma bilan kasallanish va o'lim tendentsiyalari va prognozlari - Amerika Qo'shma Shtatlari, 1982-2030". MMWR. Kasallik va o'lim bo'yicha haftalik hisobot. 64 (21): 591–6. PMC 4584771. PMID 26042651. Arxivlandi asl nusxasidan 2017-05-31.
- ^ "CDC - teri saratoni bo'yicha statistika". www.cdc.gov. Arxivlandi asl nusxasidan 2017-04-10. Olingan 2017-04-10.
- ^ "Melanoma teri saratoni uchun asosiy statistika". www.cancer.org. Arxivlandi asl nusxasidan 2017-04-10. Olingan 2017-04-10.
- ^ Urteaga O, Pack GT (1966 yil may). "Melanomaning qadimiyligi to'g'risida". Saraton. 19 (5): 607–10. doi:10.1002 / 1097-0142 (196605) 19: 5 <607 :: AID-CNCR2820190502> 3.0.CO; 2-8. PMID 5326247.
- ^ Bodenxem DC (oktyabr 1968). "Janubiy-G'arbiy mintaqada kuzatilgan 650 ta malign melanomani o'rganish". Angliya qirollik jarrohlar kolleji yilnomalari. 43 (4): 218–39. PMC 2312310. PMID 5698493.
- ^ Laennec RT (1806). "Sur les melanoses". Bulletin de la Faculté de Medecine de Parij. 1: 24–26.
- ^ Norris V (oktyabr 1820). "Fungoid kasalligi holati". Edinburg tibbiyot va jarrohlik jurnali. 16 (65): 562–565. PMC 5830209. PMID 30332089.
- ^ Norris V. Sakkizta melanoz kasalligi, ushbu kasallik bo'yicha patologik va terapevtik mulohazalar. London: Longman; 1857 yil.
- ^ Kuper S (1844). Jarrohlik nazariyasi va amaliyotining birinchi yo'nalishlari: shu jumladan printsipial operatsiyalar. SS va W. Wood.
- ^ Merriam-Vebster, Merriam-Vebsterning kollegial lug'ati, Merriam-Vebster.
- ^ mέλaς Arxivlandi 2011-06-05 da Orqaga qaytish mashinasi, Genri Jorj Liddell, Robert Skott, Yunoncha-inglizcha leksika, Perseyda
- ^ Elsevier, Dorlandning tibbiyotga oid illyustratsion lug'ati, Elsevier.
- ^ Houghton Mifflin Harcourt, Ingliz tilining Amerika merosi lug'ati, Houghton Mifflin Harcourt.
- ^ "Melanoma uchun klinik rivojlanishdagi dorilar". Farmatsevtika tibbiyoti. 26 (3): 171-83. 2012 yil 23-dekabr. doi:10.1007 / BF03262391. S2CID 13247048.
- ^ Sotomayor MG, Yu H, Antonia S, Sotomayor EM, Pardoll DM (2002). "Xatarli melanoma uchun gen terapiyasining yutuqlari". Saraton kasalligini nazorat qilish. 9 (1): 39–48. doi:10.1177/107327480200900106. PMID 11907465.
- ^ Dadli ME, Yang JK, Sherri R, Xyuz MS, Royal R, Kammula U va boshq. (2008 yil noyabr). "Metastatik melanomali bemorlar uchun qabul qiluvchi hujayra terapiyasi: intensiv miyeloablativ xemoradiatsion tayyorgarlik rejimlarini baholash". Klinik onkologiya jurnali. 26 (32): 5233–9. doi:10.1200 / JCO.2008.16.5449. PMC 2652090. PMID 18809613.
- ^ Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itjaki O, Xershkovits L va boshq. (2010 yil may). "Metastatik melanoma bilan kasallangan bemorlarda qisqa muddatli o'stirilgan o'simta infiltratsiyasi limfotsitlarini qabul qilish yo'li bilan o'tkazilgan II bosqichli tadqiqotda klinik javoblar". Klinik saraton tadqiqotlari. 16 (9): 2646–55. doi:10.1158 / 1078-0432.CCR-10-0041. PMID 20406835. S2CID 32568.
- ^ "Gen terapiyasining yangi usuli rivojlangan melanomani davolash uchun immunitet hujayralarini o'zgartiradi; bu usul boshqa keng tarqalgan saraton kasalliklariga ham tegishli bo'lishi mumkin". 2015 yil 30-dekabr. Arxivlandi asl nusxasidan 2006 yil 28 sentyabrda.
- ^ "Immunitet tizimi melanoma bilan kurashishni o'rgatdi". CBS News. 2009 yil 30-may. Arxivlandi asl nusxasidan 2010 yil 29 oktyabrda.
- ^ Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J va boshq. (Iyun 2011). "melanoma rivojlangan bemorlarda gp100 peptid vaktsinasi va interleykin-2". Nyu-England tibbiyot jurnali. 364 (22): 2119–27. doi:10.1056 / NEJMoa1012863. PMC 3517182. PMID 21631324.
- ^ Harmon A (2010 yil 21 fevral). "Davolash uchun roller coaster ta'qib qilish". The New York Times. Arxivlandi asl nusxasidan 2017 yil 10 fevralda.
- ^ a b Pollack A (2011 yil 5-iyun). "Giyohvand moddalar rivojlangan melanomani sekinlashtirishni va'da qilmoqda". Nyu-York Tayms. Arxivlandi asl nusxasidan 2017 yil 31 yanvarda.
- ^ Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J va boshq. (Iyun 2011). "BRAF V600E mutatsiyasi bilan melanomada vemurafenib bilan yashash qobiliyati yaxshilandi". Nyu-England tibbiyot jurnali. 364 (26): 2507–16. doi:10.1056 / NEJMoa1103782. PMC 3549296. PMID 21639808.
- ^ "GSK melanoma preparatlari AQSh dori-darmonlarining umumiy sonini oshiradi". Reuters. 2013 yil 30-may. Arxivlandi asl nusxasidan 2015 yil 24 sentyabrda.
- ^ "Dabrafenib va trametinib kombinatsiyasi MM kasalliklarida davolanish qarshiligini rivojlanishini kechiktiradi". Yangiliklar tibbiyot. 2012 yil 1 oktyabr. Arxivlandi asl nusxasidan 2013 yil 14 mayda.
- ^ Flaherty KT, Infante JR, Daud A, Gonsales R, Kefford RF, Sosman J va boshq. (2012 yil noyabr). "BRAF V600 mutatsiyalari bilan melanomada BRAF va MEK kombinatsiyalangan inhibatsiyasi". Nyu-England tibbiyot jurnali. 367 (18): 1694–703. doi:10.1056 / NEJMoa1210093. PMC 3549295. PMID 23020132.
- ^ "Dabrafenib / Trametinib kombinatsiyasi rivojlangan melanoma uchun tasdiqlangan". OncLive. 2014 yil 9-yanvar. Arxivlangan asl nusxasi 2014 yil 25 yanvarda. Olingan 4-fevral, 2015.
- ^ Shirley M (2018 yil avgust). "Encorafenib va Binimetinib: Birinchi global tasdiqlar". Giyohvand moddalar. 78 (12): 1277–1284. doi:10.1007 / s40265-018-0963-x. PMID 30117021. S2CID 52020890.
- ^ "Melanomada giyohvandlik qarshiligiga qarshi kurash". 2015. Arxivlandi asl nusxasidan 2015-02-04.
- ^ "Bristol preparati rivojlangan melanomada o'lim xavfini kamaytiradi". Reuters. 2010 yil 5-iyun. Arxivlandi asl nusxasidan 2010 yil 9-noyabrda.
- ^ "Bristol uchun xavf". Forbes. Arxivlandi asl nusxasi 2011-03-15.
- ^ "3-bosqich klinik tadkikoti: Ipilimumab melanoma o'smalariga qarshi immunitet ta'sirini kuchaytiradi va kuchaytiradi". News-medical.net. 2010-06-09. Arxivlandi asl nusxasidan 2012-10-19. Olingan 2012-08-13.
- ^ Jefferson E (2011-03-25). "FDA terining so'nggi bosqichidagi saraton kasalligini davolashning yangi usulini tasdiqladi" (Matbuot xabari). AQSh oziq-ovqat va farmatsevtika idorasi (FDA). Arxivlandi asl nusxasidan 2011-03-27. Olingan 2011-03-25.
- ^ Pollack A (2011-03-25). "Melanomani davolashga mo'ljallangan dori-darmonlarni tasdiqlash". The New York Times. Arxivlandi asl nusxasidan 2011-04-01. Olingan 2011-03-27.
- ^ "Yervoy". Drugs.com. Arxivlandi asl nusxasi 2011-08-09.
- ^ Robert S, Tomas L, Bondarenko I, O'Day S, Veber J, Garbe S va boshq. (Iyun 2011). "Ipilimumab plyus dakarbazine, ilgari davolanmagan metastatik melanoma uchun". Nyu-England tibbiyot jurnali. 364 (26): 2517–26. doi:10.1056 / NEJMoa1104621. PMID 21639810.
- ^ Voit S, Van Akkooi AC, Schäfer-Hesterberg G, Schoengen A, Kovalczyk K, Roewert JC va boshq. (2010 yil fevral). "Ultratovush morfologiyasi mezonlari melanomali bemorlarda qo'riqchi tugunlarining metastatik kasalligini bashorat qiladi". Klinik onkologiya jurnali. 28 (5): 847–52. doi:10.1200 / JCO.2009.25.7428. PMID 20065175.
- ^ "malignant-melanoma.org". Arxivlandi asl nusxasi 2011-10-14 kunlari.
- ^ Forbes NE, Abdelbary H, Lupien M, Bell JC, Diallo JS (sentyabr 2013). "Onkolitik viroterapiyani yaxshilash uchun o'sma epigenetikasini ekspluatatsiya qilish". Genetika chegaralari. 4: 184. doi:10.3389 / fgene.2013.00184. PMC 3778850. PMID 24062768.
- ^ Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J va boshq. (Sentyabr 2015). "Talimogene Laherparepvec rivojlangan melanoma bilan og'rigan bemorlarda uzoq muddatli reaktsiya darajasini yaxshilaydi". Klinik onkologiya jurnali. 33 (25): 2780–8. doi:10.1200 / JCO.2014.58.3377. PMID 26014293. S2CID 23663167.
Tashqi havolalar
| Axtarish, izlash melanoma Vikilug'at, bepul lug'at. |
| Vikimedia Commons-ga tegishli ommaviy axborot vositalari mavjud Melanoma. |
| Tasnifi | |
|---|---|
| Tashqi manbalar |